Fast healing among highlights to be shared at North American Spine Society’s annual meeting.
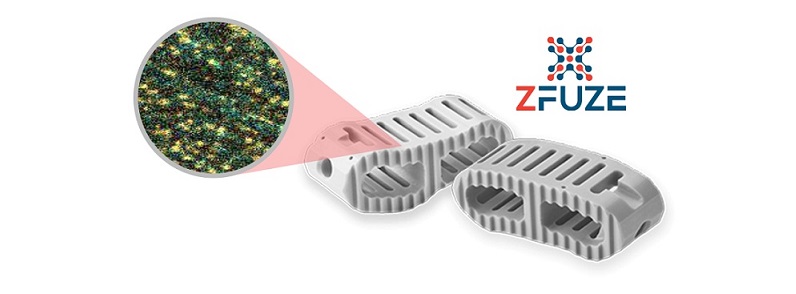
AUSTIN, Texas, Oct. 4, 2023 /PRNewswire/ — DiFusion Technologies, an industry leader in advanced biomaterials for surgical implants, announced today the results of a recent case series noting the benefits of the company’s proprietary ZFUZE™ technology. ZFUZE is the first FDA-cleared advanced biomaterial for spinal implants that is proven to create a highly favorable cellular and immunologic response post-surgery. Among a number of impressive findings, the case series showed ZFUZE offers:
- 92.3% fusion rate at 8 months
- 0% device migration
Representatives for DiFusion Technologies will share more findings and be available for interviews at the North American Spine Society’s (NASS) 38th annual meeting and conference in Los Angeles this October. Conference attendees are invited to visit booth 702 to speak with Company CEO Derrick Johns. “NASS is known on a global scale for its reputation as the most influential spine organization,” confirmed Mr. Johns. “We’re excited to participate, but even more excited for the 600,000 patients who undergo a spinal fusion each year. With ZFUZE, many of those procedures will require less downtime and have fewer complications,” he added.
Founded in 1985, NASS is the largest and most respected medical society for providers who specialize in spine care. The organization boasts a membership of more than 8,000 medical professionals, including orthopedic surgeons, neurosurgeons, and researchers. The event brings together prominent speakers, clinicians, medical societies, and other industry leaders with the goal of fostering the highest quality, ethical, value-based, and evidence-based spine care through education, research, and advocacy.
About DiFusion Technologies
DiFusion Technologies, Inc. is an industry leader in advanced biomaterials for surgical implants. DiFusion is revolutionizing the field of implant technology by developing innovative solutions that reduce the foreign body response from the patient’s immune system, thereby promoting early healing, reducing complications, and improving patient outcomes. The company’s proprietary manufacturing process produces implants that decrease chronic inflammation currently associated with mainstream biomaterials.
Media contact:
KNB Communications
Corrie Fisher
difusiontech@knbcomm.com
772-812-3823
SOURCE DiFusion Technologies, Inc.