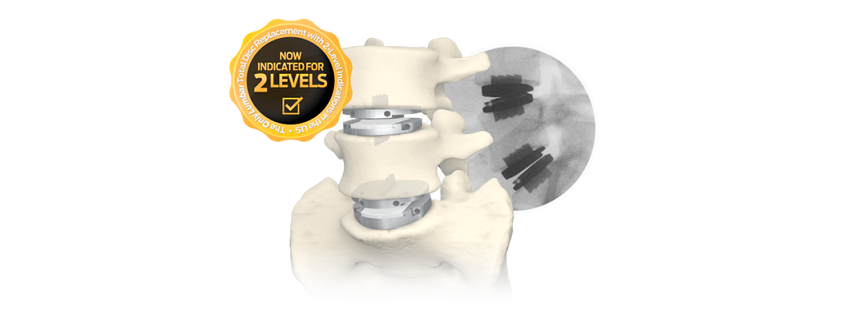
Tallahassee, FL – January 17, 2023 /OrthoSpineNews/—VySpine, a spine innovation leader using differentiated materials and designs, announced today that it has received 510(k) clearance from the FDA for its LumiVy NanoVy Ti Lumbar IBF System which is indicated for intervertebral body fusion for use at either one level or two contiguous levels in the lumbar spine, from L2 to S1, for the treatment of degenerative disc disease (DDD) with up to Grade 1 spondylolisthesis.
The LumiVy NanoVy Ti Lumbar IBF System features VySpine’s NanoVy Ti coating technology in partnership with Implant Surfaces. NanoVy Ti is a titanium coating that is mere nanometers thick, adhered to a PEEK LumiVy implant. The NanoVy Ti coating is engineered to facilitate direct osteoblast attachment to the entire surface of the LumiVy implant.
The high adhesion and impact resistant NanoVy Ti coating bonds intimately to the PEEK structure, and has been shown to increase expulsion resistance of the LumiVy implant. Furthermore, the NanoVy Ti technology does this without compromising the radiolucency, or favorable modulus of elasticity, of the PEEK material.
The LumiVy NanoVy Ti Lumbar IBF System is a robust, yet elegantly simple system. It is offered in a vast array of footprints for nearly any lumbar interbody approach. Additionally, it is available in a range of sizes, heights, and lordotic angles.
About VySpine
VySpine was created through active internal development and the licensing of various proven technologies using innovative materials and designs. This comprehensive line of core spine products and newly developed specialty products allow us to meet the needs of both health care providers and surgeons. The company strives to outpace the competition by collaborating with key spine innovators while providing a flexible, cost-effective approach to spine care. Learn more at vyspine.com
“VySpine,” “LumiVy” and “NanoVy” are registered trademarks of VySpine.
Media Contact: Paul Williams, 310-569-0023, paul@medialinecommunications.com