Frankfurt, Germany, July 25, 2022 /OrthoSpineNews/ – curasan AG (Frankfurt, Germany), a pioneer and leading global provider of biomaterials for bone and tissue regeneration in dental and orthobiologic indications, broadens its product portfolio with CERASORB® CPC (Calcium Phosphate Cement) Bone Void Fillers for maxillofacial, orthopedic and trauma surgery.
Bone augmentation and regeneration in maxillofacial, orthopedic and trauma surgery for surgical reconstructions remains a challenge. Where natural healing processes are insufficient, enhanced bone augmentation by means of biomimetic scaffolds is needed. Existing concepts for structural and functional repair of lost bone, e. g. after trauma, infections, tumor resections, or due to skeletal abnormalities are widespread, but have limitations and often result in additional bottlenecks for patients, surgeons and the healthcare systems alike.
“By adding the ready-to-use, sterile packed, self-setting bone void filler CERASORB® CPC to curasan’s orthobiologic portfolio, we are closing an important therapy gap, by offering patients, surgeons, OR staff and healthcare providers alike a unique, time- and cost-saving as well as clinically proven and effective solution for a large number of yet unmet challenges in orthopedic-, trauma- and maxillofacial surgery”, states Dirk Dembski, CEO of curasan AG. “Following our legacy as pioneers in biosurgery, we will offer this product innovation in Europe first, followed by an extended launch in other regions of the world. We look forward to living up our pioneering role in the orthobiologic market and onboarding new distribution partners for our product portfolio, that will be further broadened with disruptive products in the upcoming months”, he continues.
Empowering fracture healing with CERASORB® CPC
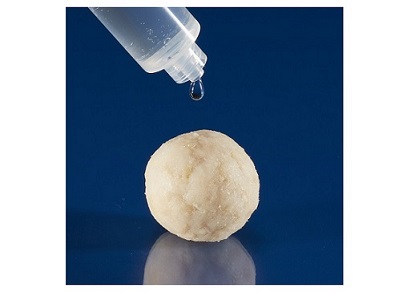
CERASORB® CPC is a synthetic, biocompatible, osteoconductive and bioresorbable mineral bone cement composed of calcium and phosphate salts finely dispersed in a biocompatible oil phase made from plant-based raw materials and emulsifiers for filling non-load-bearing and non-infected bone defects.
The setting reaction of CERASORB® CPC starts directly after application in an aqueous surgical environment, and then sets in situ to a microcrystalline, calcium-deficient hydroxyapatite (CDHA) and alpha-tricalcium phosphate. The chemical composition and crystalline structure of the final reaction product (CDHA) correspond largely to the mineral components of natural bone. Furthermore, CERASORB® CPC does not contain any substances of animal origin, added preservatives or pharmacologically active agents. The surface dimensional stability of CERASORB® CPC starts after 10 to 15 minutes and reaches a compressive strength of up to 45 MPa in a fully hardened state after several days. CERASORB® CPC is designed for use in open and minimally invasive applications, thus can be applied directly to the bone defect from the syringe or with a cannula without any further preparation.
“By introducing CERASORB® CPC we have addressed the main disadvantages of most commercially available CPC formulations, where OR personnel had to follow a strict protocol that determines the manner and timing of mixing, application, and closure of the wound” explains Florian Früh, Head of Global Marketing & Product Management at curasan AG.
“If the CPC’s were incorrectly applied, they washed-out or did not set properly, thus substantial problems raised, such as severe or more pronounced foreign body response and reduced healing. Our CERASORB® CPC bone void filler sets in vivo, and, unlike ceramic granules or blocks, can be easily formed to fill the bone defect without gaps. Moreover, the CERASORB® CPC formulation allows for a moderate bioresorbtion speed, allowing the body to replace the material by new vital bone over time or to serve as an osseous integrated material”, he concludes.
More information soon on: www.cerasorb-cpc.com
About curasan AG
curasan develops, manufactures and markets biomaterials and medical devices in the field of bone and tissue regeneration, wound healing and osteoarthritis therapy. As a pioneer and global technology leader in the growing field of regenerative medicine, curasan is specialized primarily on biomimetic bone grafting materials for dental, oral/maxillofacial, orthopaedic and spinal applications, i.e. materials mimicking biological structures. Numerous patents and a broad record of scientific publications demonstrate the clinical success of the products and the highly innovative strength of curasan. Dental and orthopaedic clinicians worldwide benefit from the broad range of the premium quality and easy to use portfolio offered by the technology leader curasan. curasan maintains its own high-tech facilities for research, development and manufacturing of biomaterials in Frankfurt/Main, Germany. In addition to its headquarters, the company has a subsidiary, curasan, Inc., in Wake Forest, near Raleigh, N.C., USA. curasan’s innovative products are cleared by the US Food and Drug Administration (FDA) and many other international authorities and available in almost 50 countries worldwide.
Press contact
Andrea Weidner
Head of Corporate Communications
Phone: +49 6027 40 900-51
Mail: pr@curasan.com
Distribution Inquiries
Jan Saipt
Sales Manager Orthobiologics
Phone: +49 6027 40 900-27
Mail: jan.saipt@curasan.com