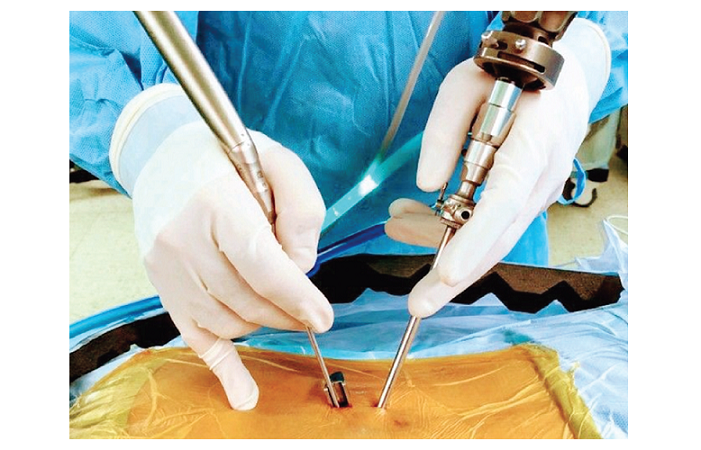
LONDON, Nov. 23, 2021 /PRNewswire/ — Smith+Nephew (NYSE:SNN; LSE:SN), the global medical technology business, today announces the launch of CORI™ handheld robotics, an advanced system for both total and partial knee arthroplasties.
The CORI system is a compact and fully mobile5 solution incorporating a 3-D intra-operative imaging system with an advanced robotic sculpting tool. The robotic system allows surgeons to measure, plan, and perform a knee surgery which is personalised to the patient’s individual anatomy in theatre.
The Smith+Nephew system is considerably more compact6 than alternative robotic systems*, has minimal set up time, and is so portable** it can be moved from theatre to theatre to optimise flow of patients through surgical units.
One of the first surgeons to use the device in the UK, Mr Tim Parratt, Consultant Orthopaedic Surgeon at East Suffolk North Essex NHS Foundation Trust says, “The CORI system is excellent to use. This technology is mobile, portable and slots seamlessly into theatre. There is minimal disruption for the theatre staff. If anything, it’s easier than getting conventional kit ready. This system also allows me to tailor the operation to the patient’s unique physiology, whereas with instruments I tended to perform the same operation every time on each patient.”
Patient Outcomes: Less pain, fewer revisions, and greater satisfaction post-surgery
Over 680,000 people are currently waiting for a hip or knee replacement7. Many of those have waited well over a year, living with chronic pain. More than 10 per cent of patients waiting for knee surgery in the UK say their quality of life is ‘worse than death’8.
The benefits of robotics-assisted surgery for patients are myriad, and include significantly improved patient reported outcome measures (PROMs)9,10 †‡ and shortened length of hospital stay11‡, there is evidence of an earlier return to an active lifestyle12‡.
Australia has been a leader in the field of robotics and navigation in orthopaedic surgery and the registry has demonstrated the benefits patients can expect such as fewer revisions13 and complications13, and a shorter length of hospital stay with the patient able to be discharged in sub-24 hours.14
“The CORI system enables every patient to have a knee replacement that is shape matched and aligned to their specific anatomy. We know that outcomes aren’t consistent in non-robotic surgeries and that one in five patients15-20 have issues following surgery. Our technology has evolved so that we can achieve a personalised fit to each individual patient,” says Mr Simon Tarry, Managing Director, UK, Ireland & Nordics, Smith+Nephew.
Innovation at Smith+Nephew
The CORI system is amongst the first technology to place the surgeon at the heart of the digital operating room. Surgeons can access the benefits of robotic surgery but still have full decision-making capability at each step of the operation.
This system is the first of several launches in technology and software over the next five years, demonstrating Smith+Nephew’s commitment to improve surgical experience through digital and technology innovation across the patient pathway.
To learn more about Smith+Nephew’s CORI surgical system please visit: www.robotics-surgery.com
*compared to Mako and ROSA®
¥compared to manual surgery techniques
**compared to NAVIO™ Surgical System
† n = 28, p<0.05, (Objective International Knee Society Score)
‡ for unicompartmental knee arthroplasty vs other techniques
πn= 28 (n=11 robotic procedures), p<0.01
References
- Batailler C, White N, Ranaldi FM, Neyret P, Servien E, Lustig S. Improved implant position and lower revision rate with robotic-assistedunicompartmental knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2019;27:1232-1240.
- Herry Y, Batailler C, Lording T, Servien E, Neyret P, Lustig S. Improved joint-line restitution in unicompartmental knee arthroplasty using a roboticassisted surgical technique. Int Orthop. 2017;41:2265-2271.
- Bollars P, Boeckxstaens A, Mievis J, Kalaai S, Schotanus MGM, Janssen D. Preliminary experience with an image-free handheld robot for total knee arthroplasty: 77 cases compared with a matched control group. Eur J Orthop Surg Traumatol. 2020;30:723-729.
- Nherera LM, Verma S, Trueman P, Jennings S. Early economic evaluation demonstrates that noncomputerized tomography robotic assisted surgery is cost-effective in patients undergoing unicompartmental knee arthroplasty at high-volume orthopaedic centres. Adv Orthop. 2020;3460675.
- Smith+Nephew 2020. CORI and NAVIO Technical Specification Comparison. Internal Report. ER0488 REV B.
- Smith+Nephew 2020. Comparison of operating room footprint for robotic-assisted knee arthroplasty systems. Internal Report. EO.REC.PCS015.002.v1.
- LCP, (14 September 2021), ‘NHS Waiting List Tracker’, https://nhswaitlist.lcp.uk.com/ (accessed: 14 September 2021).
- Clement, N. D., Scott, C. E., Murray, J. R., Howie, C. R., Deehan, D. J., & IMPACT-Restart Collaboration. (2021). The number of patients “worse than death” while waiting for a hip or knee arthroplasty has nearly doubled during the COVID-19 pandemic: a UK nationwide survey. The Bone & Joint Journal, 103(4), 672-680.
- Canetti R, Batailler C, Bankhead C, Neyret P, Servien E, Lustig S. Faster return to sport after robotic-assisted lateral unicompartmental knee arthroplasty: a comparative study. Arch Orthop Trauma Surg. 2018; 138(12):1765-1771.
- Chen K, Kim K, Vigdorchik J, Meere P, Bosco J, Iorio R. Cost-effectiveness analysis of robotic arthroplasty. Lonner JH, editor. Robotics in Knee and Hip Arthroplasty: Springer; 2019
- Sephton BM, Bakhshayesh P, Edwards TC, Ali A, Kumar Singh V, Nathwani D. Predictors of extended length of stay after unicompartmental knee arthroplasty. J Clin Orthop Trauma. 2020;11(2)S239-45.
- Canetti R, Batailler C, Bankhead C, Neyret P, Servien E, Lustig S. Faster return to sport after robotic-assisted lateral unicompartmental knee arthroplasty: a comparative study. Arch Orthop Trauma Surg. 2018; 138(12):1765-1771.
- Richard N. de Steiger, Yen-Liang Liu, Mapp and Stephen E. Graves. Computer Navigation for Total Knee Arthroplasty Reduces Revision Rate for Patients Less Than Sixty-five Years of Age. Journal of Bone and Joint Surgery. Vol 97-A, Number 8. April 15 tats, 2015.
- Sephton BM, De la Cruz N, Shearman A, Nathwani D. Achieving discharge within 24h of robotic unicompartmental knee arthroplasty may be possible with appropriate patient selection and a multi-disciplinary team approach. J Orthop. 2020;19:223-228.
- Banerjee S, Cherian JJ, Elmallah RK, Jauregui JJ, Pierce TP, Mont MA. Robotic-assisted knee arthroplasty. Expert review of medical devices. 2015;12(6):727-735.
- Urish KL, Conditt M, Roche M, Rubash HE. Robotic Total Knee Arthroplasty: Surgical Assistant for a Customized Normal Kinematic Knee. Orthopedics. 2016;39(5):e822-827.
- Nodzo SR, Carroll KM, Mayman DJ. Disposable Navigation for Total Knee Arthroplasty. Am J Orthop (Belle Mead NJ). 2016;45(4):240-245.
- Abdel MP, Oussedik S, Parratte S, Lustig S, Haddad FS. Coronal alignment in total knee replacement: historical review, contemporary analysis, and future direction. Bone Joint J. 2014;96-B:857–62.
- Stiehl JB, Komistek RD, Cloutier JM, Dennis DA. The cruciate ligaments in total knee arthroplasty: a kinematic analysis of 2 total knee arthroplasties. J Arthroplasty. 2000;15:545-550.
- Moro-oka TA, Muenchinger M, Canciani JP, Banks SA. Comparing in vivo kinematics of anterior cruciate-retaining and posterior cruciate-retaining total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2007;15:93-99.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology business that exists to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 18,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Advanced Wound Management and Sports Medicine & ENT.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $4.6 billion in 2020. Smith+Nephew is a constituent of the FTSE100 (LSE:SN,NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of COVID-19, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of COVID-19; economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers (including, without limitation, as a result of COVID-19); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of COVID-19); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew’s most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew’s expectations.
™ Trademark of Smith+Nephew. Certain marks registered US Patent and Trademark Office.
SOURCE Smith & Nephew plc