February 1, 2021
RICHARDSON, Texas–(BUSINESS WIRE)–Fuse Medical, Inc. (OTCPINK: FZMD) (“Fuse” or the “Company”) an emerging manufacturer and distributor of innovative medical devices for the orthopedic and spine marketplace, announced today an agreement with Orthovestments, LLC for the exclusive manufacturing and commercialization of the Orbitum™ Staple System (“OrbitumTM”) for the US market. This is the latest addition to Fuse’s comprehensive orthopedic portfolio of internal fixation devices for upper and lower extremities. Initial launch of the Orbitum™ is anticipated for the beginning of the second quarter of 2021.
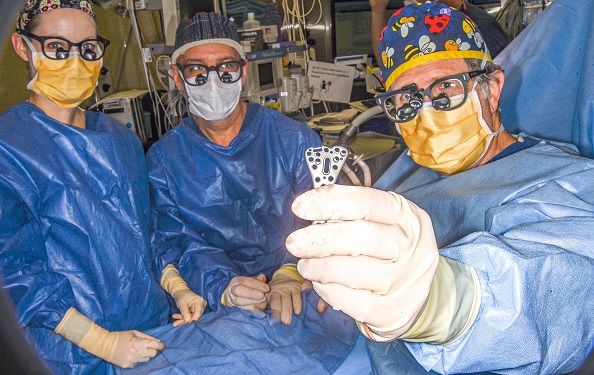
The patented Orbitum™ Staple System, which is indicated for use in the foot or hand, is a significant departure from legacy bone staple fixation. The industry’s current designs revolve around staple leg compression, yet the actual need is to achieve bone compression. The design of the titanium alloy Orbitum™ implant is novel in shape, with multiple narrow legs arranged in a radius around a central bridge. This unique leg geometry, consisting of multiple narrow tines on the inferior aspect of the implant, increases the surface area of the device. These key differentiators have demonstrated to be particularly helpful when space is limited, or if used in cancellous bone, or even when the patient exhibits soft bone density.
“We are excited to bring to market an innovative new way to provide compression in small bone fracture management with streamlined insertion techniques” commented Christopher C. Reeg, Chief Executive Officer of Fuse. “While there are many staples on the market, we believe that Orbitum™ offers a novel compression mechanism relying on physics rather than the elasticity of memory metal alloys and eliminates the concern for patients with nickel allergies.”
Orbitum™ employs a unique method for insertion into decorticated or softer bone using a punch tool, while retaining a traditional drill template and drill bit for harder bones. This gives the surgeon flexibility when implanting into different regions of the body, and in most cases, substantially cuts down operative time by reducing or eliminating the need for power instrumentation, while minimizing removal of critical bone material essential for friction seating.
Orbitum™’s templating system allows the surgeon to visually confirm size and orientation prior to the actual placement of the implant, demonstrating higher accuracy of sizing and staple leg insertion angles to further enhance the implant’s efficiency and surgical outcome. Moreover, the Orbitum™ implant leg geometry promotes superior pull out resistance five times greater than comparable staples.*
Inventor and Surgeon, Robert Weinstein, DPM, FACFAS stated “When I employ current bone staples in fusions and osteotomies, I consistently see gapping of the far cortex. This can be attributed to leg convergence which seems to induce a deleterious stress in the interface. Since the objective is to compress bone ends symmetrically, top to bottom, the Orbitum™ Staple System solves this problem, and fundamentally changes the way bone staples should be designed.”
Reeg further added, “As we continue to add innovative products to our comprehensive portfolio, our priority at Fuse remains, to provide effective solutions for today’s clinical challenges, and assist with improving surgical outcomes.”
About Fuse Medical, Inc.
Fuse is an emerging manufacturer and distributor of innovative medical devices for the orthopedic and spine marketplace. We provide a comprehensive portfolio of products in the orthopedic total joints, sports medicine, trauma, foot and ankle space, as well as, degenerative and deformity spine, osteobiologics, wound care, and regenerative medicine products. For more information about the Company, or if you’re interested in becoming a distributor of any Fuse’s products, please contact us at info@fusemedical.com or visit: www.fusemedical.com.
About Orthovestments, LLC.
Orthovestments, a collective of individuals committed to bringing elegant and effective solutions to operating room problems, was born from the concept that necessity is the mother of invention. Their current development efforts include streamlining operative techniques through innovative instrumentation and implants.
*data on file with Fuse
Forward Looking Statements
Certain statements in this press release, constitute “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “might,” “will,” “should,” “believe,” “expect,” “anticipate,” “estimate,” “continue,” “predict,” “forecast,” “project,” “plan,” “intend,” or similar expressions or statements regarding intent, belief, or current expectations, are forward-looking statements. While the Company believes these forward-looking statements are reasonable, undue reliance should not be placed on any such forward-looking statements, which are based only on information available to the Company as of the date of this release. These forward-looking statements are based upon current estimates and assumptions and are subject to various risks and uncertainties, including, without limitation, those set forth in the Company’s filings with the Securities and Exchange Commission; the failure of the Company to close the transaction; and integration issues with the consolidated company. Thus, actual results could be materially different. The Company expressly disclaims any obligation to update or alter statements whether as a result of new information, future events, or otherwise, except as required by law.
CONTACTS
Fuse Medical, Inc.
Attention: Devon Morgan, Sr. Investor Relations Analyst
1565 North Central Expressway, Suite 220
Richardson, Texas 75080
Office (469) 862-3030
Facsimile (469) 862-3035
info@Fusemedical.com