MRI clearances received in connection with certification of conformity with EU and UK Medical Device Regulations
DUBLIN, July 9, 2024–(BUSINESS WIRE)–Mainstay Medical Holdings plc today announced that it has received regulatory approvals in the European Union, the United Kingdom and Australia for full-body MR conditional labeling for the ReActiv8® Restorative Neurostimulation system. As a result, all current and future ReActiv8 patients in Europe and Australia implanted with the commercially available 45 cm leads have the ability to undergo 1.5T full-body scans. Specific scan conditions and safety information are provided in the ReActiv8 MRI Guidelines manuals for each territory.
The MRI approvals were achieved in connection with Mainstay receiving certificates issued by its Notified Body confirming conformity with the Medical Device Regulations (MDR) of both the European Union and the United Kingdom.
“These MRI approvals will allow us to significantly broaden access to ReActiv8 for patients in Europe and Australia who may require (or develop the need for) MRI imaging post-implantation, complementing our earlier approval of MRI labeling in the United States,” stated Jason Hannon, Chief Executive Officer of Mainstay Medical. “In addition, we are proud of our continued commitment to patient safety and product quality, as shown by our being among the first in the neuromodulation field to achieve MDR certification for Europe and the UK.”
Customer information emails will be sent, and MRI Guidelines and related information will be available, next week.
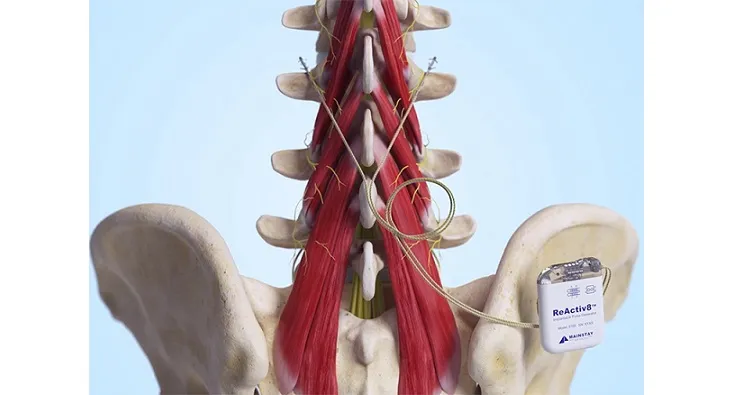
About ReActiv8®
ReActiv8 is an implantable medical device designed to treat adults with intractable chronic low back pain (CLBP) associated with multifidus muscle dysfunction. Multifidus muscle dysfunction may be evidenced by imaging or physiological testing in adults who have failed therapy including pain medications and physical therapy, and who are not candidates for spine surgery. ReActiv8 has received regulatory approval in several geographic areas, and is commercially available in the European Economic Area, Australia, the UK, and the US.
About Mainstay Medical
Mainstay Medical is a medical device company focused on commercializing its innovative implantable Restorative Neurostimulation system, ReActiv8, for people with disabling mechanical CLBP. Mainstay Medical is headquartered in Dublin, Ireland and has subsidiaries operating in Ireland, the United States, Australia, Germany, and the Netherlands.
Further information can be found at www.mainstaymedical.com.
Mainstay Forward-Looking Statements
All statements in this announcement other than statements of historical fact are, or may be deemed to be, forward-looking statements. These forward-looking statements may include, without limitation, statements regarding the company’s intentions, beliefs or current expectations concerning, among other things, the company’s labeling for MRI compatibility, the company’s ability to help additional patients as a result of having MRI compatibility labeling, and the company’s commercial efforts and performance, results, financial position, financing strategies, product design and development, intellectual property portfolio and its scope, regulatory applications and approvals, and reimbursement arrangements.
Forward-looking statements involve risk and uncertainty and are not guarantees of future performance. Actual results may differ materially from those described in, or suggested by, the forward-looking statements. A number of factors could cause results and developments to differ materially from those expressed or implied by the forward-looking statements herein, including, without limitation, the risks and uncertainties included in the company’s Annual Report for the year ended 31 December 2022, which should be read in conjunction with the company’s public disclosures (available on the company’s website (www.mainstaymedical.com)). The forward-looking statements herein speak only as of the date of this announcement.
Contacts
Mainstay PR and IR Enquiries:
LifeSci Advisors, LLC
Brian Ritchie
Tel: + 1 (212) 915-2578
Email: britchie@lifesciadvisors.com
FTI Consulting (for Ireland)
Jonathan Neilan or Patrick Berkery
Tel. : +353 86 602 5988
Email: mainstay@fticonsulting.com
Mainstay Medical
Corporate Communications
Email: Media@mainstaymedical.com