Latest FDA 510(k) cleared implant system provides comprehensive solution for Lisfranc injuries
NORTHFIELD, Ill., Jan, 30, 2024 – OrthoSpineNews – Attendees at this year’s American College of Foot and Ankle Surgeons (ACFAS) Annual Scientific Conference in Tampa, Florida will be the first to see Medline UNITE’s new Lisfranc Plating & Screw System. This new product adds to Medline UNITE’s expansive portfolio of midfoot trauma and reconstruction implants.
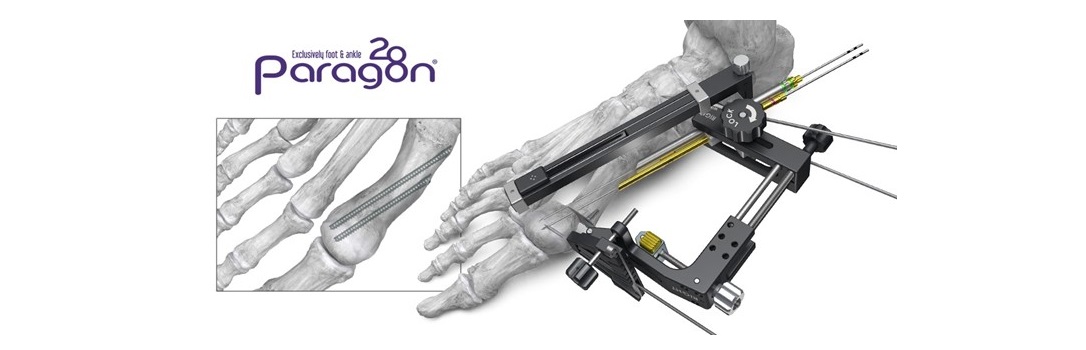
Lisfranc injuries can occur in a variety of ways from sports to motor vehicle accidents, and can result in sprains, fractures and dislocations. Patients may require surgery, resulting in either a joint fusion or internal fixation of the affected bones and ligaments until the injury has healed. The Medline UNITE Lisfranc Plating & Screw System offers 18 dual-ray Lisfranc plates that feature bendable and removable tabs to accommodate variations in patient anatomy. The plates also feature more screw holes along with a reinforcement strut design for greater stabilization of the injury.
“Our priority in introducing this plating and screw system is to help address the shortcomings of existing products on the market, particularly patient fit,” said Scott Goldstein, director of marketing for Medline UNITE Foot & Ankle. “Beyond the plates, UNITE becomes the first in the industry to offer indication-specific solid type II anodized titanium Lisfranc screws for greater strength than cannulated screws or general cortex screws. The combination of indication-specific plates, screws and instrumentation provides surgeons with the most comprehensive system on the market to treat any patient.”
The specially designed Lisfranc screws are available in Ø3.7mm and Ø4.1mm diameter options and feature a unique corticocancellous thread pitch and an optimized head profile tailored for the anatomy and indication.
“The biggest innovation in this new system is the Lisfranc targeting guide reduction clamp,” said Dr. Jonathon D. Backus, MD of Vail, Colorado. “While implant options are important, instrumentation is often the difference-maker in terms of surgical speed, efficiency and reproducibility. This ergonomically designed instrument allows the surgeon to restore the patient’s anatomy and perform every step of implanting what we refer to as a ‘homerun screw’ through a small medial incision.”
Learn more about the complete Medline UNITE portfolio at the 2024 ACFAS Annual Scientific Conference Booth #1009 or visit www.medlineunite.com. Stay up to date on the latest developments by following the Medline UNITE Foot & Ankle LinkedIn page.
About Medline
Medline is a healthcare company – a medical supply manufacturer, distributor, and solutions provider focused on improving the overall operating performance of healthcare. Partnering across the continuum of care, Medline helps providers to activate the clinical and supply chain resources needed to deliver their best care. With the agility to solve problems quickly and the scale to partner with providers for their sustained success, Medline is able to invest in its customers for the future and rapidly respond to a dynamically changing market with customized solutions.
Medline was most recently named to the Forbes America’s Best Large Employers and Best Employers for Women lists, and was recognized for the 13th year by Chicago Tribune as a Top Workplace. Headquartered in Northfield, Ill., Medline has 36,500+ employees worldwide and operates in over 125 countries and territories. Learn more at www.medline.com.
Contact:
Stacy Rubenstein
(847) 949-2286
srubenstein@medline.com
###