Nanovis celebrates this significant milestone as it continues to commercialize nanotechnology and improve patient outcomes.
COLUMBIA CITY, Ind., Oct. 17, 2023 /PRNewswire-PRWeb/ — Nanovis, a pioneer in nanotechnology solutions, is thrilled to announce the successful completion of its 500th surgery using the groundbreaking FortiFix™ pedicle screws. This milestone is not just a numeric achievement but a testament to the innovative application of FDA-designated nanotechnology, ensuring enhanced patient recovery and surgical success.
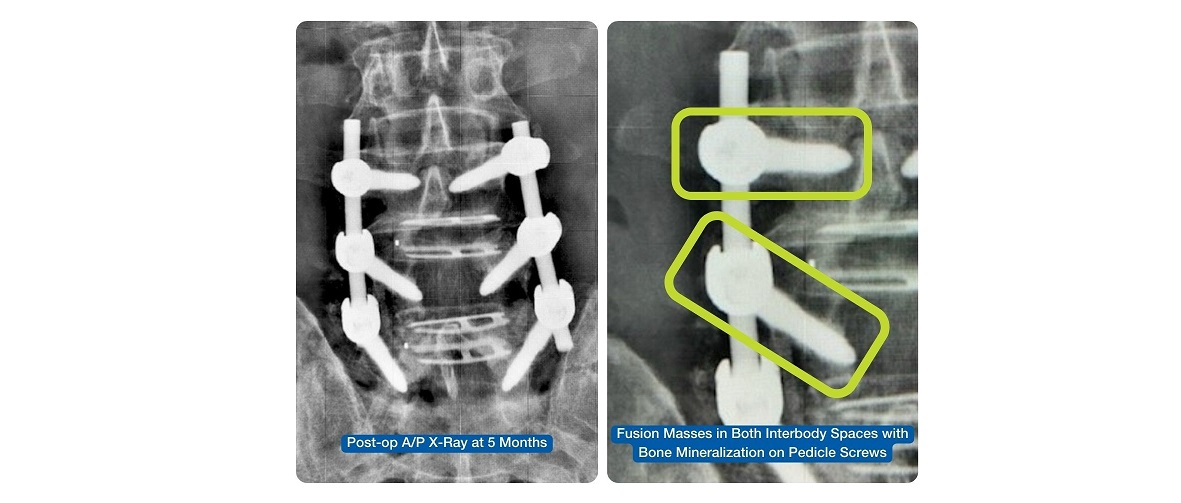
FortiFix™ pedicle screws, distinguished as the first FDA-cleared pedicle screw with a nanotechnology designation, have demonstrated a remarkable track record in facilitating biologic fixation that increases and accelerates bone-to-implant interface for osseointegration. Over 3,000 pedicle screws have been implanted in the initial 500 surgeries, with patients experiencing positive outcomes, including no known infections or non-fusions noted in the Maude database. The system has proven its efficacy across a spectrum of surgeries, including degenerative, deformity, trauma, and revision surgeries.
Dr. Todd Bonvallet, from Atlanta, GA, shares his experience: “To address the risk of pseudoarthrosis, or the failure of bone fusion to occur as intended, I have incorporated Nanovis’ nanotechnology implants to support rapid bone growth on and through the fusion construct. Utilizing Nanovis nanotechnology pedicle screws (nano FortiFix™) over the last 18 months has yielded impressive results, promoting rapid osseointegration and leading to faster and more complete fusion.”
Nanovis’ commitment to advancing spinal surgery with nanotechnology is further echoed by surgeon Dr. Wade Ceola, from Glenwood Springs, CO, who has witnessed firsthand the positive impact of nano FortiCore™ interbodies in the cervical spine over several years, and now, nano FortiFix™ pedicle screws in the lumbar spine. “The early bone ongrowth observed with the use of FortiFix™ pedicle screws not only improves healing but also ensures stronger fixation, enhancing the overall stability and success of spinal surgeries.”
“Our vision is to Enable and Engage – Enable innovation that will Engage health care and patients. With over a decade of research, we have demonstrated the biological advantages of our nanoVIS Ti Surface Technology™. Since 2019, we have had 7 spinal devices cleared by the FDA with a nanotechnology designation, including the first ever PEEK Titanium Hybrid Interbodies (nano FortiCore™) and the first ever Open and MIS pedicle screw systems (nano FortiFix™). Since the clearance of our nano FortiFix™ pedicle screw systems, I am astounded by the positive feedback we get from surgeons on their experience with the product and how the nanotechnology is helping patients heal,” states Nanovis CEO, Brian More.
More continued with his statement saying, “Medtronic’s implementation of nanotechnology, as evidenced in their recent clearance of their nanoLOCK™ surface technology on pedicle screws, validates our position that nanotechnology is clinically advancing beyond the interbody space. In this rapidly changing world of device surface technology, we should not be satisfied with traditionally manufactured implants when our nanoVIS Ti Surface Technology™ solution is truly making a difference in the lives of patients.”
Nanovis will be showcasing the nano FortiFix™ pedicle screws at the NASS 38th Annual Meeting in Los Angeles, CA, at Booth #1111 from October 18-21, 2023.
To read more about Dr. Bonvallet’s experiences with nanoVIS Ti Surface Technology™, click here.
To read more about Dr. Ceola’s experiences with nanoVIS Ti Surface Technology™, click here.
Ready to learn more about the science behind nanoVIS Ti Surface Technology™? Join us on LinkedIn.
About Nanovis
Nanovis is a technology-driven company committed to discovering unmet clinical needs, developing innovative and enabling solutions, and validating new technologies for clinical and market acceptance. The company, through its subsidiary Nanovis Spine, has successfully commercialized its proprietary nanoVIS Ti Surface Technology™ across a range of spinal implants, including the nano FortiFix™ Pedicle Screws and Nano FortiCore™ PEEK Titanium Hybrid Interbodies, the latter being enriched with OsteoSync™ technology licensed from Sites Medical.
Notably, the nanoVIS Ti Surface Technology™ platform is showing potential in pre-clinical studies to evolve into an antimicrobial surface in select markets, while concurrently, Nanovis is innovating a next-generation antimicrobial technology aimed at combating localized infections across various applications, including skin, transcutaneous devices, and orthopedic implants.
Media Contact
Guillaume Viallaneix
MedTech Momentum
1 (407) 960-2994
guillaume@medtechmomentum.com, medtechmomentum.com
SOURCE Nanovis