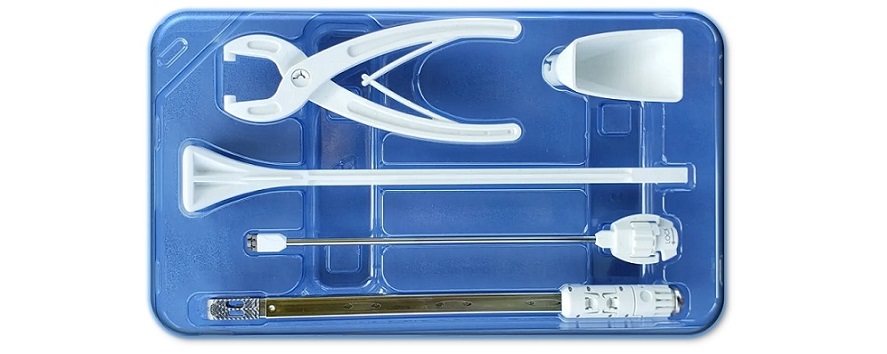
INCLINE VILLAGE, NV., Oct. 9, 2023 /PRNewswire/ — Kleiner Device Labs today announced that it was recently awarded a U.S. patent for its new KG®2 Surge® flow-thru interbody system. This adds to the company’s growing global portfolio of 35 issued patents and other intellectual property for spinal surgical technology developed by the company.
Founder and CEO Jeff Kleiner, MD, said, “We set out from the beginning to create answers for surgeons to maximize spinal fusion bone graft delivery and solve many of the most frustrating parts of spinal fusion procedures that negatively impact both the patient and the surgeon. Tabulating the results of our recently completed first 50 cases, we found that it took surgeons an average of only 8 minutes to trial, implant, graft and release the lumbar interbody device with the KG2 Surge system, and that both the mean and median volume of graft delivered was 10ml.”
“The technology that we developed with the KG2 Surge system simplifies the post-insertion delivery of a wide range of graft materials through the interbody and into the surrounding disc space,” added CTO Alan Burkholder. “Surgeons have successfully delivered milled local autograft, cortical cancellous chips, long-fiber allografts, and DBM putties.
“The simplified delivery is accomplished by marrying a rectangular inserter tube with a 3-D printed titanium interbody with a large matching opening and a bifurcated ramp that distributes the graft bilaterally. That enables filling both the cage and surrounding disc space.”
The new KG2 Surge flow-thru interbody system was developed with the objectives of maximizing bone graft delivery to the prepared intervertebral disc space, and streamlining implant placement, positioning, and integration in the graft matrix. A summary of the first 50 case results is available upon request.
For KG2 videos and information, please go to the company’s web site.
About Kleiner Device Labs
Kleiner Device Labs is creating new instruments and devices to advance minimally invasive spine surgery and improve outcomes and costs. Kleiner Device Labs is headquartered in Incline Village, Nevada.
KG and Surge are registered trademarks of Kleiner Device Labs.
For Kleiner Device Labs:
Sales and Surgeon Contact
Bob Haugen
robert.haugen@kleinerlabs.com
M: 224.223.2118
Media Contact
Brad Samson
brad.samson@kleinerlabs.com
M: 714.955.3951
Investor Contact
Stewart Peabody
stew.peabody@kleinerlabs.com
M: 312.498.5892
MKT-00010-69 Rev 1
SOURCE Kleiner Device Labs