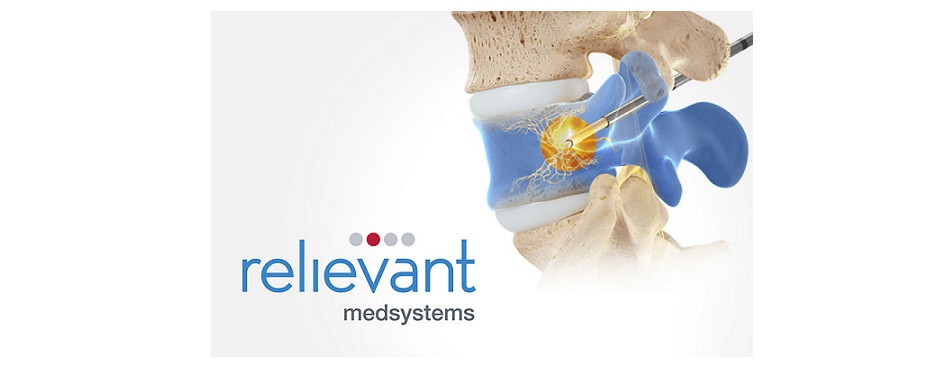
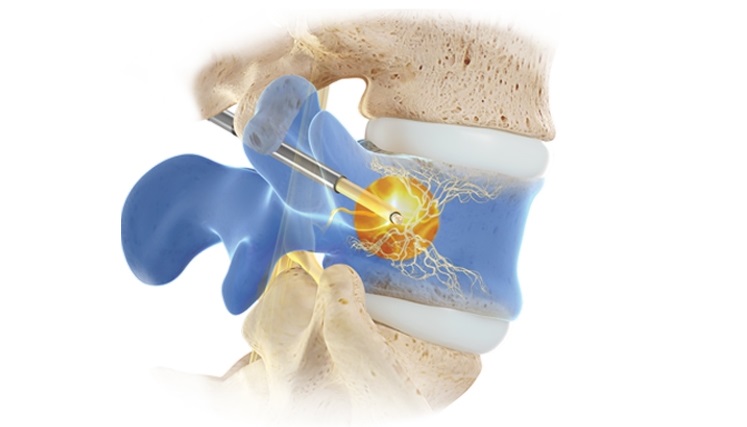
MIAMI, July 14, 2023 (GLOBE NEWSWIRE) — ASPN Annual Conference – Relievant Medsystems, a company dedicated to transforming the diagnosis and treatment of vertebrogenic low back pain, today announced the release of next-generation Intracept® Access Instruments. The new instruments offer more predictable and precise targeting of the basivertebral nerve (BVN) during the proven, durable, and safe Intracept® Procedure.
“We are committed to continued innovation in support of our physicians as we expand patient treatment with the Intracept Procedure. Today, we are pleased to announce the launch of several new instruments offering greater control, precision, and ease of use during the procedure,” said Tyler Binney, President and CEO of Relievant Medsystems. “These next-generation Intracept Access Instruments are purpose built to enhance BVN access and procedural efficiency while consistently delivering positive outcomes.”
More than 10,000 patients have been treated with Relievant Medsystems’ minimally invasive Intracept Procedure, the only FDA-cleared treatment for chronic vertebrogenic low back pain. The same-day outpatient procedure uses targeted radiofrequency energy to stop the BVN from transmitting pain signals to the brain and takes approximately one hour to perform.
The next-generation Intracept Access Instruments are designed for predictable performance across a wide range of bone densities and include:
- Bevel and Diamond Introducers to enable consistent access with a depth marker for optimal positioning
- Curved Cannula Assembly with J-Stylet that allows for true steerability to create a predictable path to the BVN
- Straight Stylet to efficiently extend or change access path trajectory
- In addition to these access instruments, the Intracept® RF Probe, which delivers targeted radiofrequency energy, now features a flexible shaft to increase C-arm operating clearance
“I have been performing the Intracept Procedure for more than a decade and I have seen the profound impact it has on improving patients’ lives,” said Gregory Moore, MD, Co-Founder and Partner of Pacific Sports and Spine. “The next-generation Intracept Access Instruments raise the bar by delivering even more predictable performance and empower physicians to more readily access and target the BVN for lasting pain relief.”
The release of the next-generation Intracept Access Instruments was announced at the Fifth Annual American Society of Pain and Neuroscience (ASPN) Conference taking place July 13-16 in Miami. For more information on the Intracept Access Instruments, visit: https://www.relievant.com/intracept/intracept-system/.
About Vertebrogenic Pain
Of the 30 million people in the U.S. with chronic low back pain, 1 in 6 are likely to have vertebrogenic pain, a distinct type of chronic low back pain caused by damage to vertebral endplates, the interface between the disc and the vertebral body. Patients typically have pain in the middle of their low back, which worsens when they bend over, sit for long periods of time, or when they are active. A physician can confirm a patient’s pain is vertebrogenic by observing Modic changes, a biomarker seen on standard MRI that indicates inflammation at the vertebral endplate.
About Relievant Medsystems
Relievant Medsystems is a commercial-stage medical device company transforming the diagnosis and treatment of vertebrogenic pain, a form of Chronic Low Back Pain (CLBP), with the Intracept Procedure – a novel, clinically proven and commercially available treatment designed to improve the quality of life for millions of indicated patients. For more information about Relievant Medsystems and the Intracept Procedure, visit www.relievant.com.
Media Contact:
Shelli Lissick, Bellmont Partners
(651) 276-6922 | shelli@bellmontpartners.com
Investor Contact:
Marissa Bych, Gilmartin Group
(805) 305-1244 | marissa@gilmartinir.com