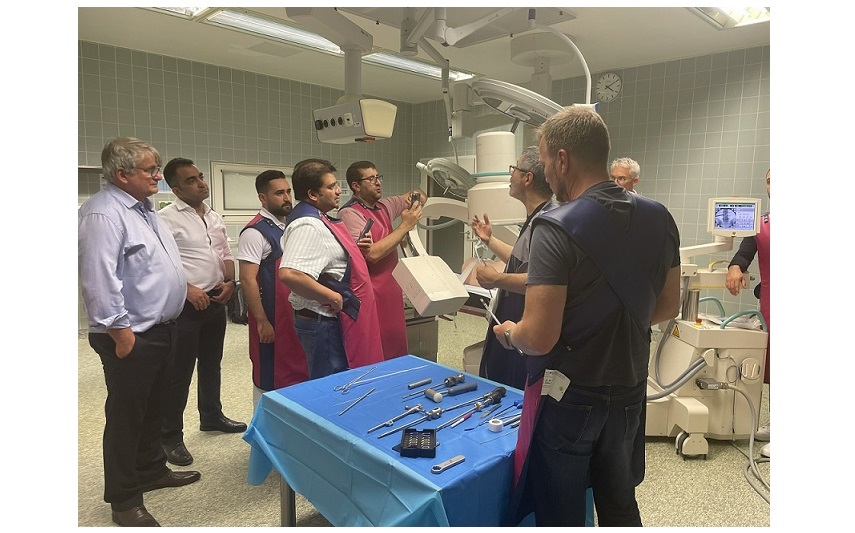
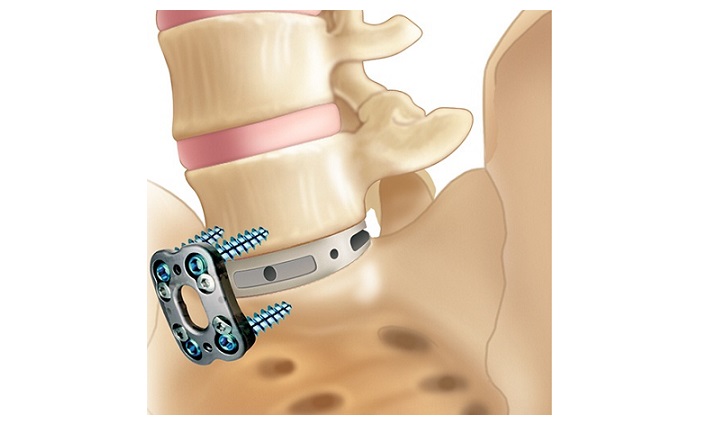
The first U.S. surgery with FDA-approved TOPS™ System, led by Jared D. Ament, MD, a board-certified and fellowship-trained neurosurgeon.
NORWALK, CONNECTICUT (PRWEB) JUNE 30, 2023 – Premia Spine, a medical technology company revolutionizing the treatment of chronic leg and back pain, today announced that the first surgical procedure using the TOPS™ System, a state-of-the-art motion preservation spinal device, has been successfully performed in the U.S., immediately following its approval by the Food and Drug Administration (FDA).
“Spinal stenosis and spondylolisthesis patients now have access to a lumbar motion device that is superior to fusion, according to its FDA label,” said Ron Sacher, CEO of Premia Spine. “We are thrilled to bring the TOPS™ System to patients who seek a non-fusion solution for their leg and back pain. We are very grateful to all the surgeons who made it possible to bring our facet replacement product to the US market, and we are particularly fortunate to have Dr. Jared Ament perform the first commercial TOPS procedure.”
The TOPS™ System is an innovative solution designed to treat spinal stenosis and spondylolisthesis. This FDA approval follows extensive clinical trials that demonstrated the system’s safety and effectiveness in alleviating back pain and improving physical function.
“This milestone is more than just a testament to our technology—it’s a testament to the hard work and dedication of our entire team,” added Peter Wehrly, President of Premia Spine. “We’re excited to see what the future holds as we continue to drive advancements in spine care.”
The groundbreaking procedure was carried out by Dr. Ament, a renowned board-certified fellowship-trained neurosurgeon at the Neurosurgery and Spine Group at the West Hills Hospital & Medical Center located in West Hills, California. This first-of-its-kind operation marks a significant milestone in the treatment of complex spine disorders, delivering hope to millions of patients suffering from debilitating leg and back pain. Premia Spine extends sincere appreciation to West Hills Hospital & Medical Center for their instrumental role in making this groundbreaking procedure possible. Their foresight and dedication to embracing cutting-edge medical technology, such as the TOPS™ System, underlines their commitment to advancing patient care. As the pioneering institution in the U.S. to offer the TOPS device, their endeavors significantly contribute to the forward momentum of spinal care nationwide. Premia Spine’s partnership with Dr. Ament and West Hills Hospital & Medical Center underscores the shared dedication to progress and patient-centered care.
“The TOPS™ technology for me is revolutionary because, until now, we’ve never had an option for posterior decompressive surgery that allows us to stabilize and not fuse. It didn’t exist. For a patient with a minor slip and significant posterior stenosis with pressure on the neural elements, we can now perform an extensive posterior decompression and open the nerve canal,” says Dr. Ament. “Until today, when you did something that extensive, you had to perform a fusion. You would put fusion rods and screws from behind and often put a cage in the disc space in front. The TOPS™ technology allows us to perform a very aggressive decompression to release the nerves, which is our primary goal for our patient and their symptoms and preserve motion. In doing so, we essentially replace the facets and the lamina, without touching the disc space, and allow mobility to be maintained. I believe TOPS will add to a patient’s quality of life—now and in the future.
When queried on his feelings about being the pioneer U.S. physician to execute the procedure following FDA’s approval, Dr. Ament expressed the following sentiment. “I’m excited. I have done many of these under the auspices of the FDA trial, so I’m comfortable with the procedure. My practice is trying to be at the cutting edge. Not to celebrate it, but to offer our patients a better solution. Being the first surgeons to use TOPS commercially is a testament to my partner, our practice, and the hospital for wanting to promote the best technology available to patients as soon as it’s available.”
The TOPS™ System, the first and only lumbar spine facet joint replacement device, provides a beacon of hope for the over 100 million individuals globally grappling with the debilitating effects of lumbar spinal stenosis and degenerative spondylolisthesis. As the only technology of its kind designed to preserve mobility post-decompression, TOPS™ offers a revolutionary alternative to the 350,000 patients annually who traditionally undergo lumbar spinal fusion. With a potential global market value of $2 billion annually, this innovation marks a transformative step forward in global spine health management.
About Premia Spine
Premia Spine, a medical technology company, is committed to enhancing the quality of life for patients suffering from chronic leg and back pain. Its products are specifically designed to offer durability, stability, and increased mobility to those struggling with lumbar spinal stenosis, degenerative spondylolisthesis, and related spinal conditions. The TOPS System, Premia Spine’s flagship product, has received the CE mark and has now obtained approval for distribution within the United States.