LAWRENCE, Kan., May 02, 2023 (GLOBE NEWSWIRE) — DirectSync Surgical, a medical device company dedicated to improving the lives of patients who suffer from spine and orthopedic diseases, has announced that its patient-powered smart spinal fusion device has received Breakthrough Device Designation by the US Food and Drug Administration.
To meet the designation criteria, FDA has reviewed the company’s preliminary data and determined that the DirectSync Surgical interbody device may provide a more effective treatment of an irreversibly debilitating condition than the current standard of care. The product delivers localized electrical stimulation and provides post-operative diagnostics to achieve fusion more effectively for patients indicated for spinal fusion. FDA’s Breakthrough Devices Program is intended to expedite development and review of medical devices that are designed to treat serious or life-threatening diseases.
“Today’s announcement is an important milestone for DirectSync Surgical and highlights the urgent need to improve spinal fusion outcomes while empowering physicians and their patients with a more robust post-operative continuum of care,” said Zygmunt Porada, CEO of DirectSync Surgical. “Obtaining breakthrough designation from FDA indicates our technology will significantly improve the standard of care and provide more timely access to patients and health care providers.”
Dr. Paul Arnold, Chairman of Neurosurgery at Carle Foundation Hospital in Illinois and Co-Founder of DirectSync Surgical said: “An all-in-one interbody fusion device that has a therapeutic aspect as well as a diagnostic tool to allow me to know when and if fusion is taking place has the potential to revolutionize my practice.”
DirectSync Surgical has successfully completed a NIH Phase II SBIR grant that supported a comparative randomized pre-clinical bone growth assessment and is currently completing a second NIH Phase I SBIR grant to integrate diagnostics to monitor key healing metrics. DirectSync Surgical is raising a Seed Round to accelerate efforts to initiate first-in-human studies.
Investment Inquiries:
Zyg Porada
phone: 443-641-6227
e-mail: zyg@directsyncsurg.com
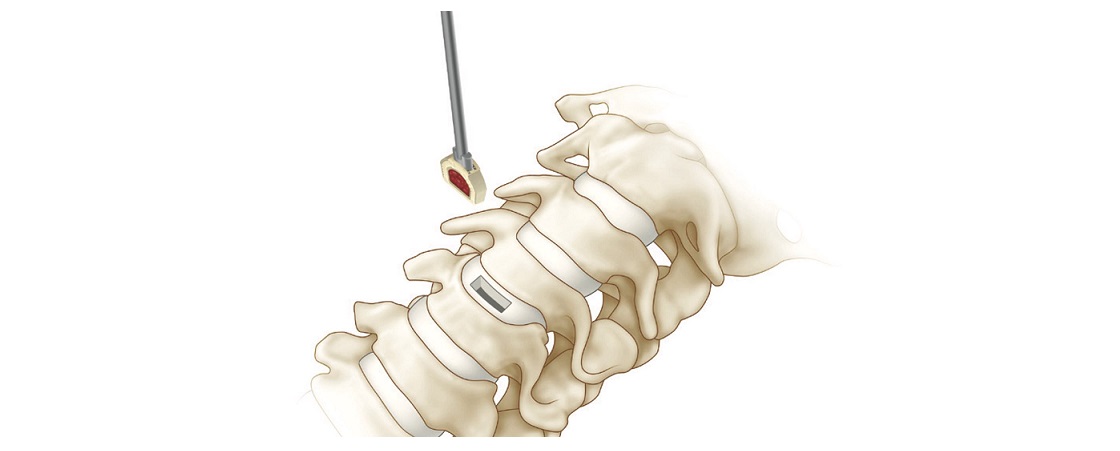
About DirectSync Surgical – DirectSync Surgical is developing the first-ever patient-powered smart implant which reduces heavy reliance on constantly powering and monitoring the device remotely. While the patient goes about their daily life, the DirectSync interbody is hard at work providing targeted mechanically synced electrical stimulation for enhanced bone healing and post-operative data collection to inform and optimize the treatment regimen between the physician and their patient.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/cbe4d054-d484-4613-bdb6-a7c8cb3dd6f2