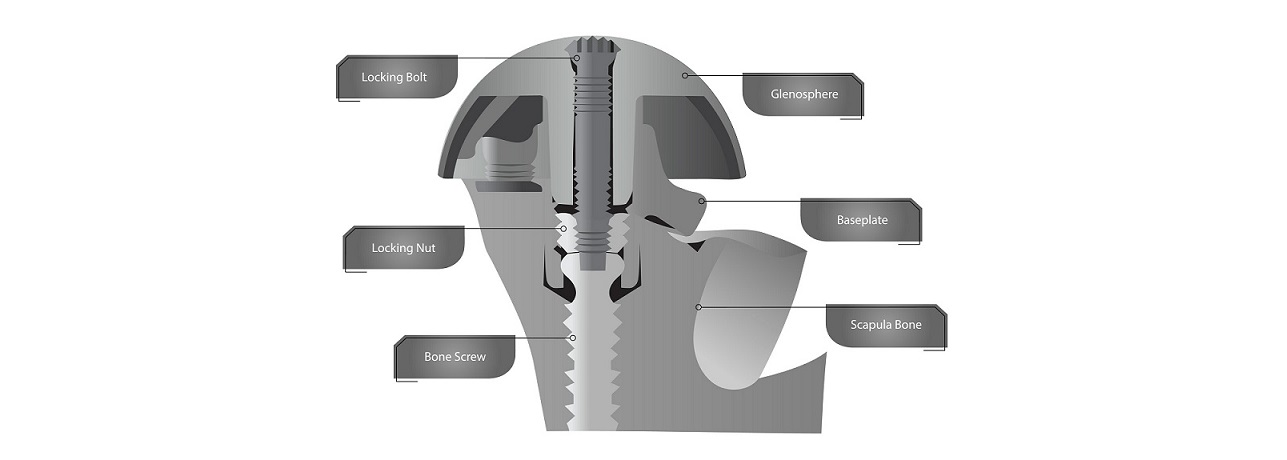
GRAND RAPIDS, Mich., April 28, 2023 /PRNewswire/ — Shoulder Innovations Inc. (SI), a Grand Rapids, Michigan based leader in the shoulder replacement implant market, announced today that in addition to its already robust portfolio of issued intellectual property, SI has received a first notice of allowance from the United States Patent and Trademark Office (USPTO) for the InSet™ Reverse System. SI is a privately-held medical device company with a broad team of successful innovators in the shoulder space. SI has developed and commercialized various components for the innovative and integrated InSet™ system, which includes the successful InSet™ Glenoid, Humeral Short Stem, Reverse and Stemless components. A platform of several technologies, the InSet Reverse System includes this first notice of allowance that has issued from USPTO for an elegant and enhanced solution to optimize potential for fixation and for intraoperative glenosphere insertion and assembly.
Rob Ball, CEO of Shoulder Innovations said, “The Shoulder Innovations team has created solutions for patients in the shoulder arthroplasty market for decades. SI released its InSet Reverse system almost two years ago, and the company has enjoyed a positive response from the marketplace. There is a significant amount of technology packaged into this system and this patent represents an important component of that technology platform. The uniqueness and value of the combination of a central bone fixation screw stabilized by a compression nut, which is in turn supported by a glenoid locking bolt, is validated by this Notice of Allowance by the USPTO.”
About Shoulder Innovations:
Shoulder Innovations is a medical device development company that designs and commercializes innovative products that demonstrate the potential for improved patient care and reduced overall cost to the healthcare system.
Leveraging its breakthrough, patented, InSet™ glenoid design, Shoulder Innovations is commercializing a shoulder replacement implant system focused on improving outcomes related to the greatest cause of shoulder replacement failure: glenoid loosening.
The InSet™ technology has been shown in testing to significantly reduce glenoid implant micro-motion and simplifies the surgical technique, potentially reducing complications or increasing implant longevity.
For more information about Shoulder Innovations and the Power of One™ visit www.shoulderinnovations.com.
Please note that the InSet™ Stemless Humeral Implant is not yet cleared for use in the United States in a reverse articular configuration.
SOURCE Shoulder Innovations