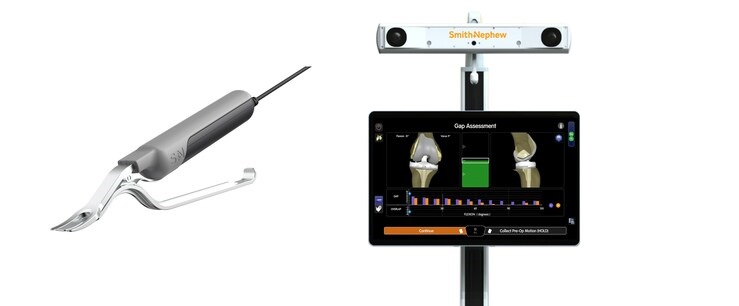
LONDON, April 24, 2023 /PRNewswire/ — Smith+Nephew (LSE:SN, NYSE:SNN), the global medical technology company, today introduced its new CORI™ Digital Tensioner – a purpose built device that lets surgeons measure the ligament tension in a knee prior to cutting bone.1 By enabling a surgeon to quantify joint laxity in the native knee and achieve an optimal ligament tensioning force, the CORI Digital Tensioner helps to reduce variability when balancing the knee in surgery.1-4 This helps make surgical planning more objective versus other commercially available alternatives.1,3
Personalizing Surgery and Helping Reduce Variability in Robotics-assisted TKA
The CORI Digital Tensioner produces a surgeon-defined, quantifiable force to distract the knee joint, apply consistent tension to the ligaments, and provide objective gap data for procedure planning and execution.1,3 A small clinical case series showed the CORI Digital Tensioner reduced variability of tensioning by 64% when compared to a manual technique.5*
“I’ve never seen anything like the digital tensioner before,” stated Dr. Steven Haas at Hospital for Special Surgery in New York. “You try to teach how much force to apply, but it varies by surgeon and from one case to another. The CORI Digital Tensioner goes into the knee, you see a force or load being applied, and the gap it created. This technology is something I’ve wanted for the last twenty years.”
Advancing procedural innovation in robotic-assisted surgery, the CORI Digital Tensioner is a differentiated technology that helps solve challenges for surgeons, including:
- The first and only ligament tensioning device in robotic-assisted surgery to assess joint laxity in the native knee before performing bony resection.
- Automatic collection of gap data at a specified force through the full range of movement.
- A true tensioning device using a software interface that allows surgeons to choose their preferred target force value (50±10N, 100±10N, or 150±10N).
The first commercial surgical procedure using the CORI Digital Tensioner was recently performed by Dr. Bertrand Kaper of Orthopedic Specialists of Scottsdale and HONORHEALTH Medical Group in Arizona.
“The integration of this dedicated soft tissue tensioning device brings a new realm of objective data to the technique of knee replacement,” commented Dr. Kaper. “The surgeon is now able to collect joint laxity data, in a proactive manner, to assist with the visualization, planning and execution of the patient’s surgery in real time. It’s unlike any other tool we’ve historically had available and will be a powerful addition to the robotic-assisted knee replacement technology that the CORI™ platform currently offers.”
To learn more about the latest advancements in orthopaedic reconstruction and discover more about robotics and computer-guided solutions, please go to https://www.smith-nephew.com/en-us/health-care-professionals/products/orthopaedics/cori
* n=4
References
- Smith+Nephew 2022. Optimus TKA Tensioner Gap Assessment Verification Report. Internal Report. 10059269.
- Smith+Nephew 2021. Tensioner Design Verification Test Report. Internal Report. TR100123
- Smith+Nephew 2022. Tensioner KPC: Tensioner Calibration Check. Internal Report. TR100116, Rev.B
- Haas SB, Chow J, Nathwani D, Belooshi A, et al. Smith+Nephew CORI™ Digital Tensioner for total knee arthroplasty (TKA): ligament tensioning and assessment prior to bone resections. S+N Evidence in Focus Whitepaper. 2022 Dec.
- Smith+Nephew 2022. Tensioner Whitepaper Supporting Evidence. Internal Report. 10073166 REV A.
About Smith+Nephew
Smith+Nephew is a portfolio medical technology company focused on the repair, regeneration and replacement of soft and hard tissue. We exist to restore people’s bodies and their self-belief by using technology to take the limits off living. We call this purpose ‘Life Unlimited’. Our 19,000 employees deliver this mission every day, making a difference to patients’ lives through the excellence of our product portfolio, and the invention and application of new technologies across our three global franchises of Orthopaedics, Sports Medicine & ENT and Advanced Wound Management.
Founded in Hull, UK, in 1856, we now operate in more than 100 countries, and generated annual sales of $5.2 billion in 2022. Smith+Nephew is a constituent of the FTSE100 (LSE:SN, NYSE:SNN). The terms ‘Group’ and ‘Smith+Nephew’ are used to refer to Smith & Nephew plc and its consolidated subsidiaries, unless the context requires otherwise.
For more information about Smith+Nephew, please visit www.smith-nephew.com and follow us on Twitter, LinkedIn, Instagram or Facebook.
Forward-looking Statements
This document may contain forward-looking statements that may or may not prove accurate. For example, statements regarding expected revenue growth and trading margins, market trends and our product pipeline are forward-looking statements. Phrases such as “aim”, “plan”, “intend”, “anticipate”, “well-placed”, “believe”, “estimate”, “expect”, “target”, “consider” and similar expressions are generally intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. For Smith+Nephew, these factors include: risks related to the impact of Covid, such as the depth and longevity of its impact, government actions and other restrictive measures taken in response, material delays and cancellations of elective procedures, reduced procedure capacity at medical facilities, restricted access for sales representatives to medical facilities, or our ability to execute business continuity plans as a result of Covid; economic and financial conditions in the markets we serve, especially those affecting healthcare providers, payers and customers (including, without limitation, as a result of Covid); price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal and financial compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers (including, without limitation, as a result of Covid); competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses; disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; relationships with healthcare professionals; reliance on information technology and cybersecurity; disruptions due to natural disasters, weather and climate change related events; changes in customer and other stakeholder sustainability expectations; changes in taxation regulations; effects of foreign exchange volatility; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Smith+Nephew has filed with the U.S. Securities and Exchange Commission under the U.S. Securities Exchange Act of 1934, as amended, including Smith+Nephew’s most recent annual report on Form 20-F, which is available on the SEC’s website at www. sec.gov, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Smith+Nephew as of the date of the statement. All written or oral forward-looking statements attributable to Smith+Nephew are qualified by this caution. Smith+Nephew does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Smith+Nephew’s expectations.
™ Trademark of Smith+Nephew. Certain marks registered in US Patent and Trademark Office.
SOURCE Smith & Nephew plc