Recent clinical study indicates positive data on Amplify Surgical’s dualLIF® System, dualPortal® Endoscopic TLIF with dualX®
IRVINE, CALIF. (PRWEB) APRIL 17, 2023 – Amplify Surgical, Inc., a medical device company focused on innovative minimally-invasive surgery for the lumbar spine, today announced positive data from a retrospective study on its dualLIF® System, the synthesis of dualPortal® Spinal Endoscopy and dualX® Dual-Expanding Interbody Technology. The study, “The Use of Dual Direction Expandable Titanium Cage With Biportal Endoscopic Transforaminal Lumbar Interbody Fusion: A Technical Consideration With Preliminary Results,” was recently published in Neurospine, led by clinical researchers, Don Y. Park, M.D., Vice Chair of Quality and Safety of the Department of Orthopaedic Surgery, David Geffen School of Medicine at UCLA in Los Angeles, California and Dong Hwa Heo, M.D., Ph.D., Director of the Department of Neurosurgery, Spine Center, Champodonamu Spine Hospital in Seoul, South Korea. Link: https://pubmed.ncbi.nlm.nih.gov/37016859/
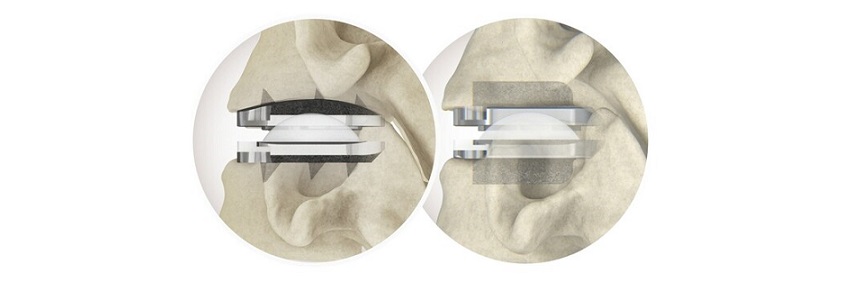
The first description of dualLIF® in the scientific literature, the study analyzed 10 patients on whom dualLIF® was performed, over 6 months after surgery. Mean VAS leg scores, back scores, and ODI all significantly improved. Additionally, mean intervertebral disc heights and lumbar lordosis both increased notably. There were 0 incidental durotomies, wound infections, implant failures, or medical complications in this clinical series. Furthermore, postoperative radiographs at 6 months demonstrated no malposition, significant subsidence, or re-collapse of inserted cages. Results from the study indicate the efficacy of dualLIF® (dualPortal® endoscopic TLIF using the dualX® dual-expanding cage) as a successful surgical technique for treating lumbar degenerative disease.
Dr. Don Y. Park notes, “This technical note marks the first ever publication that describes the dualLIF® procedure in detail and demonstrates favorable early clinical experience in the United States. We showed significant improvement in clinical outcome scores and sustained radiographic improvement over a 6 month follow up after surgery. Importantly, there were no significant instances of subsidence or loss of correction in the early postoperative period, possibly due to the wide footprint and design of the dualX® cage. These results provide support that the dualLIF® endoscopic procedure can be effective in the early follow up period.”
Referred in the study as the dual direction expandable titanium cage, Amplify Surgical’s dualX® technology is a fleet of titanium, expandable interbody devices designed to expand in both width and height, implanted in lateral lumbar interbody fusion (LLIF), posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF) spinal procedures. The dualX® portfolio contains varying footprints, heights, and degrees of lordosis with post-expansion bone grafting to provide an optimal anatomical fit for a clinically successful fusion environment. The dualPortal® technique is Amplify Surgical’s novel two-portal endoscopic approach to the spine that allows surgeons to easily learn and perform a wider array of lumbar spine procedures than the conventional one-portal technique. Also referred to as (unilateral) biportal endoscopy, dualPortal™ provides flexibility to perform endoscopic lumbar fusions with conventional expandable cages or dualX®. Amplify Surgical’s dualLIF® synthesizes dualPortal® Spinal Endoscopy and dualX® Dual-Expanding Interbody Technology, integrating the enhanced visualization of endoscopic spine surgery with the advantages of minimally-invasive TLIF and dual-expanding interbody cages.
About Amplify Surgical, Inc.
Amplify Surgical is a privately-held spinal device company located in Irvine, CA. The company’s mission is to revolutionize minimally-invasive spine surgery by transforming ordinary procedures with groundbreaking surgical solutions that improve patient safety and outcomes.