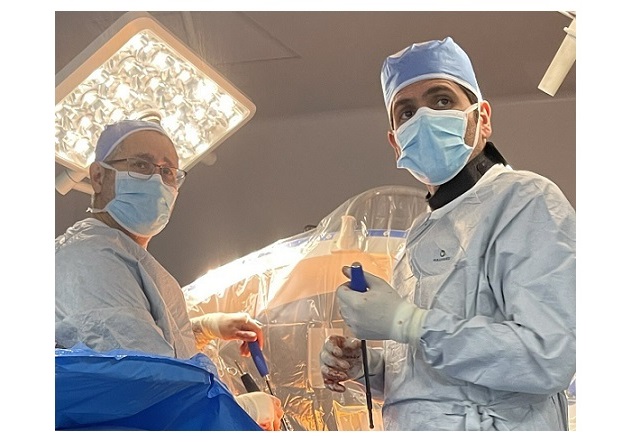
CHATTANOOGA, Tenn., March 30, 2023 /PRNewswire/ — 3Spine, Inc., today announced completion of the 50th MOTUS lumbar total joint replacement in the United States under FDA Investigational Device Exemption (IDE) and the 150th Real-World Evidence (RWE) lumbar fusion control case. This second milestone marks full enrollment of the RWE fusion study, allowing all eligible sites to recruit MOTUS patients upon conversion.
“Our incredible enrollment progress highlights the strength of our clinical research organization,” said Marc Peterman, co-founder and CEO of 3Spine. “At this stage for a Class III technology, it’s expensive and talent really matters. The RWE fusion study was a heavy lift. Surgeons had to enroll their fusion cohort before starting the IDE and, as you might imagine, there just wasn’t a lot of excitement around a spinal fusion study. Well, our team got it done. Now everyone is converting to MOTUS, and new patients are motivated to participate. People are actively searching for an alternative to fusion. It’s amazing to see the difference.”
“I think what you are seeing in the study is a good predictor of what will happen in the market,” said Jeff Goldstein, MD, national Principal Investigator (PI) for the IDE. “Patients naturally delay spinal fusion until their symptoms are sometimes very advanced, which is a different conversation when we are talking about a reconstructive alternative. There may be an advantage to intervene earlier in the disease process, while tissues are healthier and remobilization is easier.”
Dom Coric, MD, national PI for the RWE fusion study, continued, “I tell my fusion patients, ‘Come back and see me when you can’t take it anymore.’ With MOTUS I think the conversation will be more like, ‘Come back and see me when you can’t run your next race.’ MOTUS has the potential to change when and how we look at surgical intervention.”
3Spine’s lumbar total joint replacement procedure and MOTUS device is a ‘first-of-kind’ technology replacing the function of the disc and facet joints through a posterior approach. Much like a hip or knee total joint replacement, the technique reconstructs the functional spinal unit. The procedure is intended to broadly address leg pain, back pain, and spinal instability, while correcting posture and preserving freedom of movement in patients suffering from spinal stenosis, facet arthritis, and other degenerative changes. Lumbar total joint replacement received FDA Breakthrough Device Designation in 2020 and is currently tracked under CPT code 0719T.
The 150-patient RWE cohort will be propensity score matched to a 150-patient IDE cohort at two years. U.S. approval is expected in 2025. 3Spine is seeking single-level indications from L1-S1 in patients suffering from lumbar degeneration with or without foraminal or recess spinal stenosis with no more than a grade 1 spondylolisthesis at the involved level. Additional details are available at ClinicalTrials.gov.
About 3Spine
3Spine, Inc. is a private, clinical phase healthcare company headquartered in Chattanooga, Tennessee, dedicated to advancing patient care through the development of lumbar (low back) total joint replacement as an alternative to spinal fusion. With a goal to radically transform spine surgery, the company’s breakthrough MOTUS lumbar total joint replacement is designed to preserve natural balance, retain range of motion and preserve posture. 3Spine is changing the standard of care for spine patients to ensure they can live a more active, pain-free life. To learn more, follow us on LinkedIn, Facebook and Twitter, or visit www.3spine.com.
SOURCE 3Spine