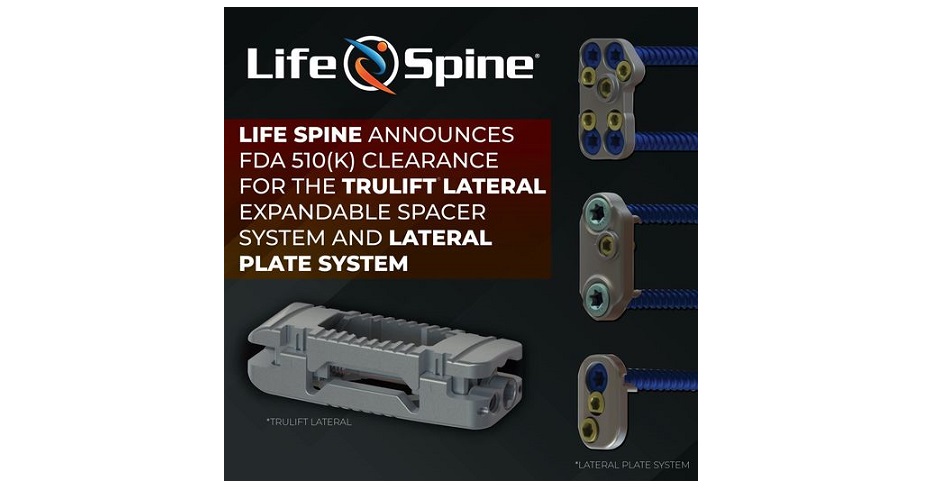
HUNTLEY, Ill., December 14, 2022 –(BUSINESS WIRE)–Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the TruLift Lateral Expandable Spacer System and Lateral Plate System.
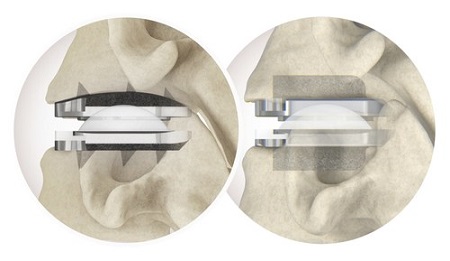
Utilizing expandable technology and robust plating options, TruLift Lateral and Lateral Plate System provide physicians with unique possibilities for their patient care. TruLift Lateral is offered in a range of sizes and footprints and can be expanded to the desired height (8mm to 16mm) to suit the patients’ individual pathology and anatomical conditions. The 1-screw, 2-screw, and 4-screw Lateral Plates provide immediate stabilization and fixation of the lumbar spine and may optionally attach to the TruLift Lateral Expandable Spacer.
“TruLift Lateral and Lateral Plate System offer next-generation technology for Lateral Lumbar Interbody Fusions. The system features a robust titanium, expandable interbody that allows for plate fixation devices to attach easily and accurately. These plates are offered in multiple configurations and sizes. Separately or combined, these innovative systems further our commitment to Micro Invasive Procedures,” said Rich Mueller, Chief Operating Officer of Life Spine.
TruLift Lateral Features and Benefits:
- Post-packable & repositionable, in-situ
- Up to 8mm of in-situ expansion and disc height restoration
- Minimized impaction, preserved end plate integrity, and designed to reduce psoas irritation
Lateral Plates Features and Benefits:
- Audible, tactile and visual confirmation of CAM locking mechanism provides confidence in preventing screw back out
- Plate teeth provide intraoperative stabilization and prevents postoperative plate rotation
- Optional connection screw to fasten the plate to the interbody minimizes risk of implant migration
About Life Spine
Life Spine is dedicated to improving the quality of life for spinal patients by increasing procedural efficiency and efficacy through innovative design, uncompromising quality standards, and the most technologically advanced manufacturing platforms. Life Spine, which is privately held, is based in Huntley, Illinois. For more information, please visit: http://www.lifespine.com and/or https://www.micro-invasive.com.
Contacts
Mr. Omar Faruqi
Chief Financial Officer
ofaruqi@lifespine.com
847-884-6117