PALM BEACH GARDENS, Fla., Nov. 03, 2022 (GLOBE NEWSWIRE) — Accelus, a privately held medical technology company focused on accelerating the adoption of minimally invasive surgery (MIS) as the standard of care in spine, today announced that the U.S. Food & Drug Administration (FDA) had cleared Accelus’s 510(k) application for its Remi™ Robotic Navigation System software update that allows image capture with the GE OEC 3D, Ziehm Vision RFD 3D, and Stryker Airo TruCT systems.
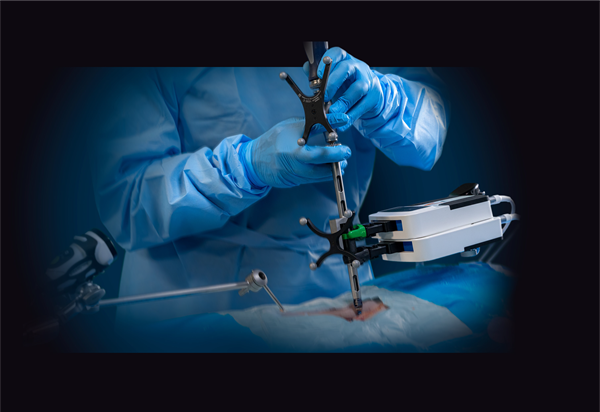
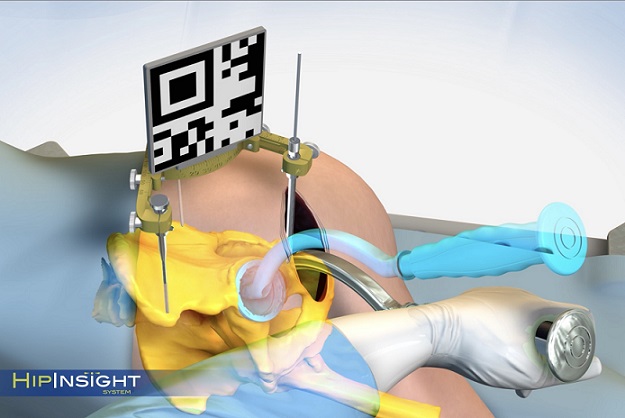
The Remi Robotic Navigation System is a robotic targeting and navigation platform that provides robotic-assisted pedicle screw placement for surgeons performing lumbar spine fixation. It was cleared by the FDA in February 2021 with patient anatomy and fiducials identified through an O-arm scan. The new FDA clearance allows the system to be used with a broader portfolio of 3D imaging systems, which can reduce the barriers often associated with robotic navigation.
“Cost, footprint and complexity were previously unscalable barriers that kept robotic spine surgery from being performed anywhere but at large hospitals; however, the Remi system’s new clearance provides smaller facilities and ambulatory surgery centers (ASCs) with access to its disruptive technology that can help precisely navigate for accurate screw placement with reduced reliance on traditional fluoroscopy,” Chris Walsh, Accelus’s Chief Executive Officer and Co-Founder, said. “We are proud to have achieved this next step as we look to fulfilling the remaining unmet needs in spinal robotic navigation and are excited to now be able to offer our Remi Robotic Navigation System to so many more facilities and surgeons.”
The Remi system was designed to address the inherent limitations of legacy robotic systems in spine, including extended setup and teardown time, high costs, procedural workflow disruptions and taking up a large footprint in the operating room (O.R.). This new clearance enhances the advantages of the Remi system by allowing it to be used with a multitude of 3D imaging systems, lessening the need for hospitals to invest in a specific imaging platform.
“The Remi system addresses the concerns of previous spinal navigation systems with equivalent accuracy and an optimized procedural workflow at a fraction of the cost. The ability to combine it with the GE OEC 3D system is game-changing for my practice,” said Ernest Braxton, MD, MBA, neurosurgeon at Vail-Summit Orthopaedics and Neurosurgery. “Being able to utilize robotic navigation in an ASC outpatient setting provides me with the precision I need with less radiation exposure for my patients, my O.R. team, and me. It’s a win-win-win.”
All Remi Robotic Navigation Systems in the field will be updated to the newly cleared software and will also continue to be compatible for use with O-arm imaging.
About Accelus
Accelus is committed to accelerating minimally invasive spine surgery through its enabling technology with broad accessibility to previously underserved markets. Established in 2021 through the combination of Integrity Implants and Fusion Robotics, the company is focused on providing its proprietary Adaptive Geometry™ technology with pragmatic and economical navigation and robotic solutions with broad clinical use in spine surgery. Learn more at www.accelusinc.com.
Disclaimer
GE, Ziehm and Stryker are not affiliated with Accelus and nothing in this press release is intended to imply a relationship with, or endorsement or sponsorship of Accelus or its products by, these or any other companies.
Media Contact: Brandy Craig
305-676-1679
bcraig@accelusinc.com