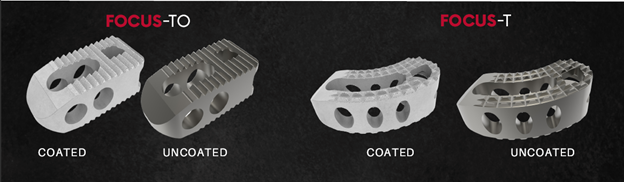
Savannah GA, October 18, 2022 /OrthoSpineNews/ – OC Spine a division of OC Medical Devices a privately held medical device company focused on developing, manufacturing, and commercializing spinal implants that address unmet clinical needs, announced today the FDA has given 510(k) approval of its Focus family of Ti TLIF interbody devices featuring their xCELLerate bioactive surface technology.
Jason Bazemore VP of Sales & Marketing states “We are excited to have clearance of the Focus line and also thrilled that this paves the way for incorporating our xCELLerate technology on future implant systems in our development pipeline”. Focus will be available in both oblique and banana style options and is cleared for use with or without the xCELLerate technology. The hydrophilic nature of xCELLerate is shown to attract and absorb blood increasing the growth factors needed to promote the integration of bone to the implant.
OC Spine is a division of OC Medical Devices headquartered in Savannah, GA. OC Spine delivers the highest quality spinal implants and instruments that are derived from surgeon input. We develop innovative solutions produced with German manufacturing precision. OC Spine’s goal is to provide sterile implant solutions with the objective of improving patient outcomes.
Media Contact
Jason Bazemore
Vice President of Sales & Marketing
Jason.bazemore@ocmedicaldevices.com
978.489.9020