Solidifying expertise in high-impact trauma and fragility fractures of the pelvis
September 28, 2022
BELLEVUE, Wash.–(BUSINESS WIRE) — CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, today announced the addition of three new members to its Surgeon Advisory Board. The new members bring together expertise in orthopedic traumatology, pelvis and acetabular trauma reconstruction, and Fragility Fractures of the Pelvis (FFP) to support CurvaFix’s focus on the development and commercialization of a new treatment option for pelvic fractures using the CurvaFix® IM Implant.
“The new members of our Surgeon Advisory Board represent a breadth of surgical expertise and technical knowledge across orthopedic trauma medicine, including high-impact pelvic trauma and Fragility Fractures of the Pelvis,” said Steve Dimmer, chief executive officer for CurvaFix. “These thought leaders bring outstanding research and clinical credentials to CurvaFix’s focus on offering a new approach to pelvic fracture repair that provides strong, stable, curved fixation with a simpler, minimally invasive procedure with the potential to immediately reduce pain, allow for earlier mobility and improve patient recovery compared to traditional surgical techniques,” continued Dimmer.
The newest members of CurvaFix’s Surgeon Advisory Board are:
- Samir Mehta, M.D. is a Philadelphia-based orthopaedic trauma surgeon at a busy Level I University Hospital. He holds several local, national, and international leadership positions in orthopaedic trauma, helping define the goals of care for the next generation of surgeons and volunteers in developing nations providing education and patient care.
- Prof. Dr. med. Dr. h.c. Pol Maria Rommens is a retired Head of the Department of Orthopedics and Traumatology at the University Medical Center of the Johannes Gutenberg-University of Mainz, Germany. Professor Rommens is currently an AO Trauma International Board member and chairperson of the AO Trauma Research Commission. He has authored 675 original papers, including Comprehensive Classification of Fragility Fractures of the Pelvic Ring: Recommendations for Treatment (2013), and is co-editor of the book, Fragility Fractures of the Pelvis (2017).
- G. Karl Van Osten, III, M.D. is a Director of Orthopaedic Trauma Service at the North Mississippi Medical Center in Tupelo. Dr. Van Osten has done research in fracture mechanics, reconstruction, and nail fixation. He maintains an active interest in research endeavors and is a frequent presenter in local and national surgeon education programs.
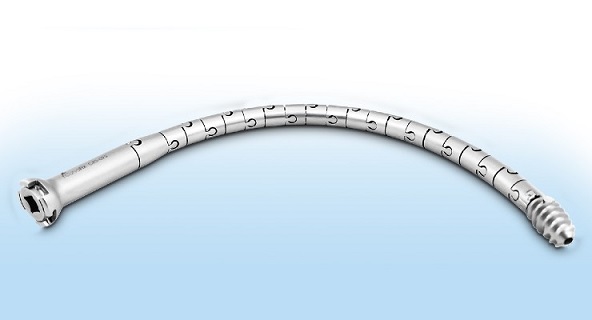
The CurvaFix IM Implant has the potential to overcome the limitations of existing implants used for pelvic fracture fixation in both high-impact trauma and fragility fracture patients. Case reports have indicated that the curved implant achieves strong, stable fixation that follows and fills the natural curvature of each patient’s anatomy, which may immediately reduce pain, allow for earlier mobility, and improve patient recovery. The novel implant enables surgeons to address challenges such as dysmorphic sacra, curved superior rami, and fragility fractures in geriatric patients with a fast, easy, and minimally invasive procedure.
There are over 186,000 hospitalizations for pelvic fractures in the U.S. every year. Due to an aging population, the incidence is growing at 9% per year. Of those hospitalized, over 108,000 are due to FFP injuries in geriatric patients. FFPs can dramatically change the quality of life for geriatric patients and their families due to a loss of patient autonomy, significant disability, and even death. Despite recommendations that surgical treatment should be considered for most pelvic fragility fractures, only 10% receive surgery today. For non-operative patients, conservative treatment generally consists of bed confinement, pain control, and mobility assistance while tolerating weight-bearing. Often, conservative treatment leads to lengthy hospitalizations, high nursing home admittance, and a high one-year mortality rate. In contrast, decades of innovation in hip fracture repair have enabled strong, stable surgical fracture fixation to become the standard of care. Ninety-five percent of hip fracture patients receive surgery today, which greatly reduces pain and often allows geriatric hip fracture patients to mobilize soon after surgery.1
About CurvaFix, Inc.
CurvaFix, Inc. is a privately held medical device company headquartered in Bellevue, Wash. CurvaFix is developing implantable products to improve fracture repair in curved bones. The company is focusing on Fragility Fractures of the Pelvis and high-impact pelvic fractures. The CurvaFix IM Implant has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
CurvaFix is a trademark of CurvaFix, Inc., registered in the U.S.
___________________________
1 Orthopedic News Network, Vol 32, No 2, May 2022
Contacts
Amy Cook
Acook@curvafix.com