Éragny-sur-Oise, Fleurieux-sur-l’Arbresle, November 25, 2021 at 5:45 pm CET – Safe Orthopaedics (FR0013467123 – ALSAF), a company specializing in the design, manufacture and marketing of ready-to-use technologies for back surgery, particularly safe for emergency-treated vertebral fractures, announces the 20,000th kit sold, and the success of the evaluation phase of Sycamore, the novel vertebral compression fracture (VCF) treatment.
Firstly, Safe Orthopaedics has successfully reached the significant mark of 20,000 instrument kits sold across the globe. With this achievement many patients have been successfully treated using Safe Orthopaedics’ proprietary ready-to-use technology that delivers significant benefits to the hospital, the surgical team, and the patient.
On this topic, Nik Beyer, Chief Commercial Officer of Safe Orthopaedics says: “Despite significant market headwinds I’m delighted that through the hard work and determination of our direct sales teams, distribution partners and support teams we have continued to prove that there is a real, and growing, un-met need for our ready-to-use technology in the global spine market.”
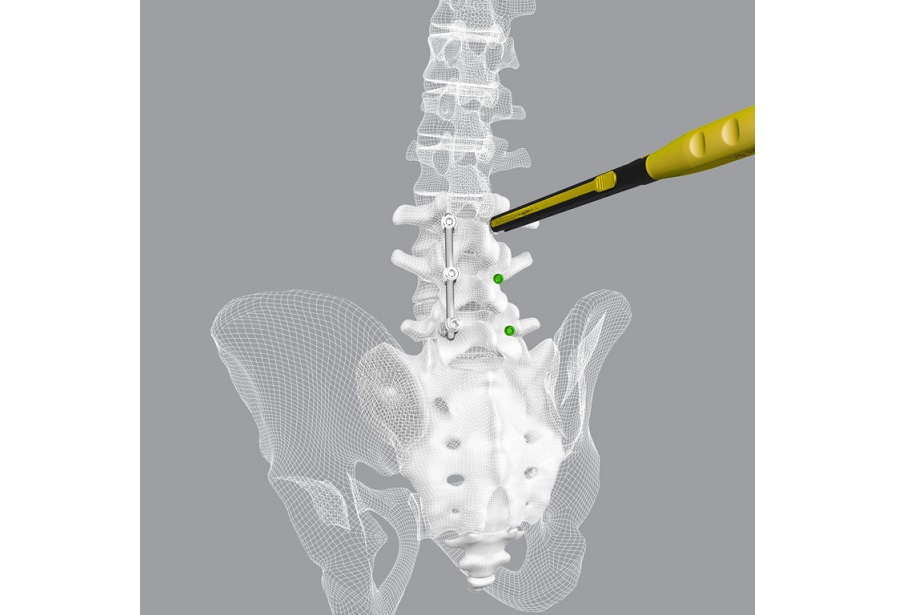
Secondly, through close collaboration with surgeons in France and Germany, Safe Orthopaedics is coming to the end of a successful evaluation phase for its novel vertebral compression fracture (VCF) treatment, Sycamore. With the completion of this phase Sycamore is ready for launch early 2022 in select markets.
Nik Beyer comments: “Sycamore is an exciting product for our portfolio, a new concept to the market that promises to deliver significant growth through 2022 and beyond in a VCF market that continues to show year-on-year growth. Market trends showed us that the solutions available to surgeons to manage these hard-to-treat patients do not always achieve the best results, Sycamore is designed to meet those demands”.
Pierre Dumouchel, CEO & Chairman of Safe Group concludes: “It’s with great pride that we make this announcement today. Our focus has always been on delivering the best possible outcome for patients and it’s a privilege to have contributed to the optimal treatment of so many patients. To deliver this milestone in tough market conditions, during a pandemic, and follow-up with a major piece of product development in Sycamore is testament to the investment we have made in both our commercial and innovation business units and the close working partnerships we have with our expert surgeons. For VCF patients, a long-term correction of the fracture is key to delivering the best patient outcome. Our Sycamore implant is designed to deliver exactly that”.
About Safe Group
Safe Group is a French medical technology group that brings together Safe Orthopaedics, a pioneer in ready-to-use technologies for spine pathologies, and Safe Medical (formerly LCI Medical), a subcontractor of medical devices for orthopedic surgery. The group employs approximately 150 people.
Safe Orthopaedics develops and manufactures kits combining sterile implants and single-use instruments, available at any time to the surgeon. These technologies are part of a minimally invasive approach aimed at reducing the risk of contamination and infection, for the benefit of the patient and with a positive impact on hospitalization times and costs. Protected by 18 patent families, SteriSpineTM kits are CE marked and FDA approved. Safe Orthopaedics is headquartered in the Paris region (Eragny-sur-Oise (95610)) and has subsidiaries in the United Kingdom, Germany, the United States and the Lyon region (Fleurieux-sur-l’Arbresle).
For more information: www.safeorthopaedics.com
Safe Medical produces implantable medical devices and ready-to-use instruments. It has an innovation centre and two production sites in France (Fleurieux-sur-l’Arbresle, 69210) and Tunisia, offering a wide range of industrial services: design, industrialisation, machining, finishing and sterile packaging. Supported by the French recovery plan in 2020, the company invests in additive printing and will be operational in 2022 on this new technology.
For more information: www.safemedical.fr
Contacts
Safe Orthopaedics
François-Henri Reynaud
Chief Financial and Administrative Officer
Tél. : +33 (0)1 34 21 50 00
investors@safeorthopaedics.com
Press Relations
Ulysse Communication
Pierre-Louis Germain / +33 (0)6 64 79 97 51 / plgermain@ulysse-communication.com
Bruno Arabian / +33 (0)6 87 88 47 26 / barabian@ulysse-communication.com