Merck plans to submit an application for Emergency Use Authorization to the FDA soon based on these findings.
October 4, 2021 / Jeff Lagasse, Associate Editor
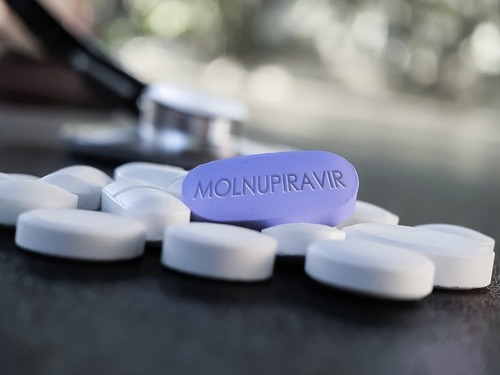
In an effort to push back against the still-ongoing global COVID-19 pandemic, Merck, in partnership with Ridgeback Biotherapeutics, is touting the benefits of an investigational oral antiviral medication known as molnupiravir, saying it reduces the risk of hospitalization and death among people with mild-to-moderate cases of the coronavirus.
At the interim analysis, molnupiravir reduced the risk of hospitalization or death by about 50%, according to Merck; 7.3% of patients who received molnupiravir were either hospitalized or died through Day 29 following randomization, compared with 14.1% of placebo-treated patients.
Through Day 29, no deaths were reported in patients who received molnupiravir, as compared to 8 deaths in patients who received placebo.
At the recommendation of an independent Data Monitoring Committee and in consultation with the U.S. Food and Drug Administration, recruitment into the study is being stopped early due to the results, the company said. Merck plans to submit an application for Emergency Use Authorization to the FDA soon based on these findings, and plans to submit marketing applications to other regulatory bodies worldwide.
Countries around the globe have taken notice. Thailand, for instance, is in talks with Merck to purchase 200,000 courses of the experimental pill, joining South Korea, Taiwan and Malaysia, according to Reuters. The Philippines is currently running a trial on the pill, and hopes a domestic study on its efficacy will eventually result in public access to the treatment.