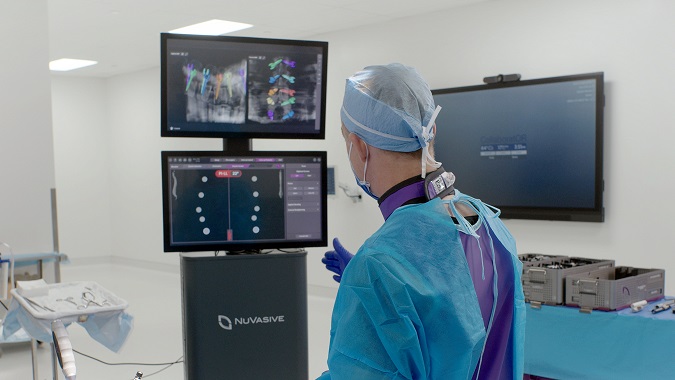
Leading U.S. surgeons and hospitals complete first commercial cases with NuVasive’s integrated enabling technology platform for spine surgery
SAN DIEGO, Sept. 30, 2021 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, today announced Drs. Michael Kachmann and Zachary Tempel from Mayfield Brain & Spine in Ohio, and Dr. Paul Holman from Houston Methodist Hospital in Texas, completed the first commercial cases with the NuVasive Pulse® platform.
Drs. Kachmann and Tempel successfully performed the first commercial case using Pulse in an XLIF® procedure with minimally invasive fixation and nerve root decompression at TriHealth’s Good Samaritan Hospital in Ohio, and have since leveraged Pulse in a variety of spine procedures. In utilizing the technology, Dr. Kachmann said, “We have spent years waiting for a spine technology platform like Pulse. The ability for multiple surgical applications to exist in a single platform and provide integrated feedback on our surgical approach from one screen was unlike anything I’ve ever utilized in spine surgery. It was incredible to see the improvements in accuracy and efficiency throughout the procedure compared to other available technologies.”
When treating his patient and reflecting on Pulse’s surgical workflow with a less invasive procedure, Dr. Tempel said, “Pulse easily complemented how our OR staff operated. It even enhanced how we were able to move from XLIF to posterior fixation with all the necessary tools in one platform, making seamless transitions between applications. Mayfield Brain & Spine has been a strong advocate for the Pulse platform and it will become an integral part of the world-class patient care we offer.”
Dr. Holman, a thought leader in spine navigation technology, had this to share after utilizing the Pulse platform, “This technology is a game changer for spine surgery—from its condensed footprint in the OR to its utility throughout the entire procedure—this will transform the standard of spine care for patients. Having helped with the clinical development of the platform, this leading technology will advance how I work and teach in the operating room, and ultimately improve patient outcomes.”
In addition, other leading surgeons and hospitals have selected Pulse as the technology platform for their respective spine operating rooms.
Pulse integrates multiple technologies into one platform: radiation reduction,1 imaging enhancement, rod bending, navigation, intraoperative neuromonitoring, and spinal alignment tools. It is the only enabling technology in spine that has the ability to be utilized in 100% of open or less invasive spine procedures.2 Pulse received its latest U.S. Food and Drug Administration (FDA) clearance and CE certification earlier this summer.
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is the leader in spine technology innovation, with a mission to transform surgery, advance care, and change lives. The Company’s less-invasive, procedurally integrated surgical solutions are designed to deliver reproducible and clinically proven outcomes. The Company’s comprehensive procedural portfolio includes surgical access instruments, spinal implants, fixation systems, biologics, software for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative neuromonitoring technology and service offerings. With more than $1 billion in net sales, NuVasive has approximately 2,700 employees and operates in more than 50 countries serving surgeons, hospitals, and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons and hospitals, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products, the Company’s ability to adequately manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
1 Wang TY, Farber SH, Perkins SS, et al. Internally randomized control trial of radiation exposure using ultra-low radiation imaging versus traditional C-arm fluoroscopy for patients undergoing single-level minimally invasive transforaminal lumbar interbody fusion. Spine 2017;42(4);217-23.
2 The Pulse platform can be used in every spine procedure; however, not all modalities are cleared for every spine procedure. Refer to Pulse system instructions for use.
SOURCE NuVasive, Inc.