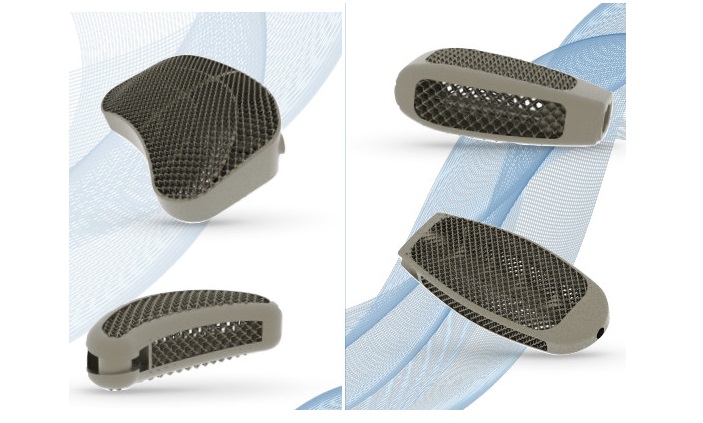
SAN DIEGO, July 22, 2021 /PRNewswire/ — NuVasive, Inc. (NASDAQ: NUVA), the leader in spine technology innovation, focused on transforming spine surgery with minimally disruptive, procedurally integrated solutions, announced today the commercial launch of Modulus® ALIF, a 3D-printed porous titanium implant for anterior lumbar interbody fusion (ALIF), in targeted global regions.
“Modulus ALIF has been one of NuVasive’s most successful clinical evaluations to date with continued surgeon adoption and overwhelmingly positive feedback. This differentiated technology furthers our position to become the market leader in ALIF and extends our Modulus portfolio across all procedural segments,” said Massimo Calafiore, executive vice president, Global Business Units at NuVasive. “We are leveraging our experience as the leader in lateral surgery to deliver outcome-driven innovation to fuel continued growth and differentiation in the anterior spine segment.”
Modulus ALIF is the latest addition to the NuVasive Advanced Materials Science® (AMS) portfolio and provides implants and instrumentation designed for both supine ALIF and XALIF™ procedures. In addition, Modulus ALIF comes in a variety of sizes and lordotic options to accommodate varying patient anatomy. Highlights of the Modulus ALIF technology include:
- Proprietary Modulus titanium design: Modulus is designed for enhanced osseointegration,1 biomechanical,1 and imaging properties. The porous surface architecture is engineered to participate in fusion and promote new bone on-growth and in-growth.2 The proprietary design also allows for enhanced visualization compared to solid titanium implants.
- Zero-step locking mechanism: This feature provides definitive tactile and visual confirmation, enabling confidence in a surgeon’s screw placement in the implant.
- Low-profile, versatile instrumentation: The device’s instrumentation can be utilized in both supine ALIF and XALIF, supporting a surgeon’s preferred surgical approach. The instrumentation’s low-profile and versatile features are designed to increase operating room workflow efficiencies.
“Modulus ALIF has been a long awaited implant in my practice. The design is best-in-class and the differentiated surface structure will help improve fusion and clinical outcomes,” said Gregory M. Mundis Jr., M.D., orthopedic spine surgeon and co-director of the San Diego Spine Fellowship at Scripps Health in San Diego. “The device’s instrumentation is designed to work with various patient anatomies. This versatility makes me more confident that I am choosing the best solution for my patients.”
NuVasive is committed to furthering innovation and clinical validation of X360®, a comprehensive lateral approach to single-position surgery that includes XLIF®, XALIF, and XFixation™. Recently, a new journal article was published in World Neurosurgery that further validates the improved benefits possible with single-position surgery compared to dual-position lumbar fusion. The findings concluded that shorter operating time and hospital stays were more prevalent in single-position surgery while maintaining similar clinical outcomes.
NuVasive will feature Modulus ALIF at these upcoming events:
- NuVasive Innovation Event: A surgeon-focused event today, July 22, where NuVasive leadership and surgeon partners will discuss NuVasive’s outcome-driven innovation including Modulus ALIF, the Pulse® platform, C360®, and more. Visit nuvasive.com/innovationevent for more information.
- 37th Annual Meeting of the Section on Disorders of the Spine and Peripheral Nerves: On Thursday, July 29, NuVasive will host a lunch workshop discussing patient-specific care with NuVasive’s ALIF, XALIF, and AMS portfolio of implants. Visit NuVasive at booth #301 where it will also feature the Pulse platform, X360, and the Simplify® Cervical Disc.
About NuVasive
NuVasive, Inc. (NASDAQ: NUVA) is the leader in spine technology innovation, with a mission to transform surgery, advance care, and change lives. The Company’s less-invasive, procedurally integrated surgical solutions are designed to deliver reproducible and clinically proven outcomes. The Company’s comprehensive procedural portfolio includes surgical access instruments, spinal implants, fixation systems, biologics, software for surgical planning, navigation and imaging solutions, magnetically adjustable implant systems for spine and orthopedics, and intraoperative neuromonitoring technology and service offerings. With more than $1 billion in net sales, NuVasive has approximately 2,700 employees and operates in more than 50 countries serving surgeons, hospitals, and patients. For more information, please visit www.nuvasive.com.
Forward-Looking Statements
NuVasive cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause NuVasive’s results to differ materially from historical results or those expressed or implied by such forward-looking statements. The potential risks and uncertainties which contribute to the uncertain nature of these statements include, among others, risks associated with acceptance of the Company’s surgical products and procedures by spine surgeons and hospitals, development and acceptance of new products or product enhancements, clinical and statistical verification of the benefits achieved via the use of NuVasive’s products, the Company’s ability to adequately manage inventory as it continues to release new products, its ability to recruit and retain management and key personnel, and the other risks and uncertainties described in NuVasive’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made.
###
| ____________________ |
| 1 Preclinical data on file; Data may not be representative of clinical results. |
| 2 Preclinical data on file; Data may not be representative of clinical results. TR 9604787. |
SOURCE NuVasive, Inc.