July 15, 2021
MEMPHIS, Tenn.–(BUSINESS WIRE)–Active Implants, LLC, a developer of orthopedic implant solutions for joint preservation, today announced two-year results of the MERCURY study, which showed that the company’s NUsurface® Meniscus Implant provides statistically superior pain relief beginning at six months compared to non-surgical therapy. The results were presented at the American Orthopedic Society for Sports Medicine-Arthroscopy Association of North America Combined 2021 Annual Meeting in Nashville.
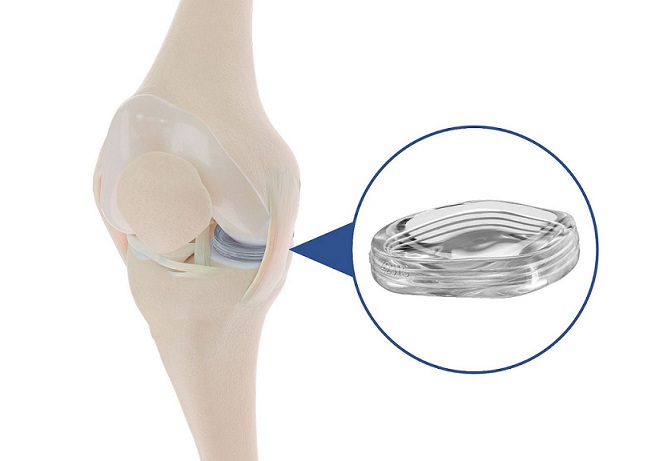
The NUsurface Implant is the first “artificial meniscus” to be marketed in Europe and is currently under review by the U.S. Food & Drug Administration (FDA). The medial meniscus replacement mimics the function of the natural meniscus and treats pain by redistributing loads transmitted across the knee joint. It was granted a Breakthrough Device Designation from the FDA in 2019.
“The MERCURY study showed that patients who received the NUsurface Implant experienced statistically superior pain relief as early as six months that continued through the two-year study period, compared to patients receiving non-surgical care alone,” said Wayne Gersoff, M.D., a MERCURY investigator from Advanced Orthopedic & Sports Medicine Specialists in Denver, who presented the study results. “There is a significant need for new treatment options for patients who have persistent knee pain following a meniscectomy, which the NUsurface Implant may address.”
The MERCURY Study is the longest and most extensive study of a meniscus implant in the world, enrolling 242 patients experiencing persistent knee pain after a previous meniscectomy: 176 were treated with the NUsurface Implant and 66 with non-surgical therapy. Results of the study include:
- 84% of patients treated with the NUsurface Implant experienced a 10-point improvement from baseline in Knee Injury and Osteoarthritis Outcome Score (KOOS) Pain, which is considered the minimum clinically relevant improvement in pain and function.
- 89% of patients who received the NUsurface Implant completed 24 months of follow-up.
- Patients with replacement implants achieved superior results the second time; 70% of patients used one implant and 20% received a replacement implant.
- Obese and older patients had the highest response rates.
- MRI evidence showed the NUsurface Implant may preserve femoral cartilage, while the control patients showed evidence of significant cartilage deterioration.
The abstract, “Superior Improvements in Knee Pain and Function with a Novel Synthetic Medial Meniscus Replacement Implant Compared to Nonsurgical Care in Subjects with Knee Pain Following Partial Meniscectomy: Two-year Results from Two Prospective US Clinical Trials” was selected to be featured in an AOSSM-AANA meeting press release.
“We’re gratified that AOSSM-AANA selected the MERCURY study results as one of the presentations to highlight – a reflection of the fact that current treatment options for post-arthroscopic partial meniscectomy knee pain are limited, especially for patients considered too young for knee replacement,” said Ted Davis, president and CEO of Active Implants. “The two-year results of the study demonstrate that the NUsurface Implant not only reduces pain but may also help preserve cartilage on the medial femoral condyle.”
About the NUsurface® Meniscus Implant
The NUsurface® Meniscus Implant is an investigational treatment for patients in the U.S. with persistent knee pain following medial meniscus surgery. It is made from medical grade polymer and, as the result of its unique materials, composite structure and design, does not require fixation to bone or soft tissues. The NUsurface Implant mimics the function of the natural meniscus and redistributes loads transmitted across the knee joint. It is currently marketed in Belgium, Germany, Italy, the UK and Israel.
About Active Implants, LLC.
Active Implants, LLC., develops orthopedic implant solutions that complement the natural biomechanics of the musculoskeletal system, allowing patients to maintain or return to an active lifestyle. Active Implants is privately held with headquarters in Memphis, Tennessee. European offices are in Haarlem, The Netherlands, with R&D facilities in Netanya, Israel. For more information, visit www.activeimplants.com. Follow the company on Twitter, LinkedIn and Facebook.
CAUTION Investigational device. Limited by United States law to investigational use.
Contacts
Joni Ramirez
Merryman Communications
joni@merrymancommunications.com
323.532.0746