FREMONT, Calif. and DRAPER, Utah, May 10, 2021 /PRNewswire/ — THINK Surgical, Inc., an innovator in the field of orthopedic active robot surgery, and Ortho Development Corporation, an orthopedic device design and manufacturing company, have announced FDA clearance to use Ortho Development’s Balanced Knee® System (BKS), and BKS TriMax® implants, with THINK Surgical’s TSolution One® Total Knee Application. The TSolution One Total Knee Application is the only commercially available active robot system for total knee replacement that supports an open implant library, giving surgeons a choice of implant options.
“We are delighted to expand the open implant library of the TSolution One Total Knee Application to include Ortho Development’s Balanced Knee System and BKS TriMax implants,” said Jay Yang, acting CEO, THINK Surgical, Inc. “Ortho Development’s reputation for attaining reproducible outcomes in total knee arthroplasty combined with the accuracy available to surgeons through the use of the TSolution One Application makes this an exciting offering for surgeons.”
The collaboration enhances each company’s pioneering technology: THINK’s TSolution One Total Knee Application active robot and Ortho Development Corporation’s Balanced Knee System and BKS TriMax, which includes implants and instrumentation for total knee replacement procedures.
Brent Bartholomew, President of Ortho Development, added, “We are optimistic about our collaboration with THINK Surgical, and the ability to offer a robotic option to our customers in this expanding area of orthopedic surgery. The BKS and BKS TriMax have an excellent clinical history and market reputation going back 20+ years. Coupling these implants with the TSolution One Total Knee Application adds a unique enabling technology for our flagship products.”
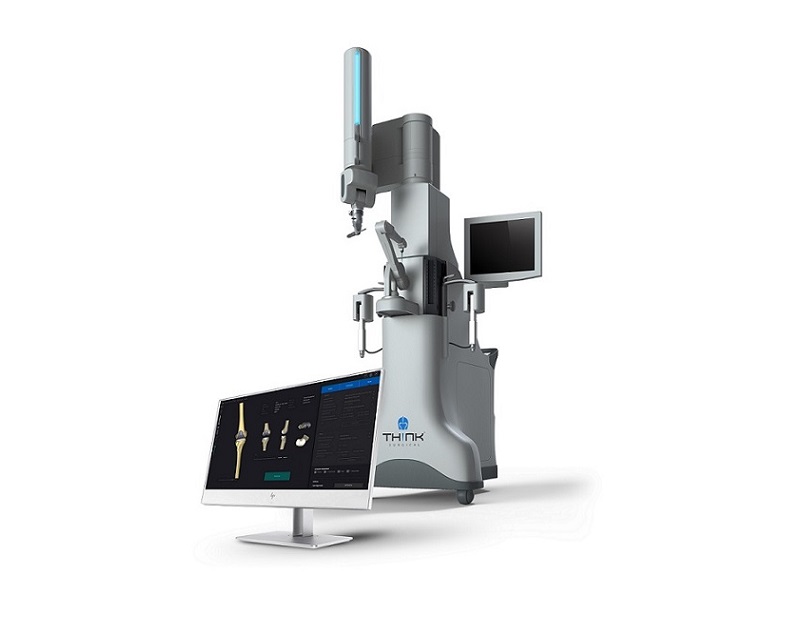
The TSolution One Total Knee Application consists of TPLAN®, the 3D pre-surgical planning workstation, and TCAT®, the active robot. Pre-surgical planning allows the surgeon to design and prepare, in a virtual environment, the patient’s personalized joint replacement surgical plan. The active robot aids the surgeon in executing the preoperative surgical plan with automated, hands-free cutting and removal of the diseased bone and cartilage. The TSolution One Total Knee Application assists surgeons with optimal joint implant placement based on each patient’s unique anatomy.
Ortho Development’s BKS and BKS TriMax design is based on proven technology with more than 20 years of successful clinical results and over 100,000 implanted worldwide. BKS implants are offered in a wide range of sizes and options to allow optimal anatomical fit for a variety of patients. The system has a patented locking mechanism specifically designed to minimize the micro-motion between the plastic tibial insert and the titanium tibial base plate implants. Simple and intuitive instrumentation facilitates reproducibility in the hands of every surgeon and aids in proper intraoperative balancing of the knee joint.
About THINK Surgical, Inc.
THINK Surgical, Inc., a privately held U.S.-based medical device and technology company, develops, manufactures, and markets active robotics for orthopedic surgery. The TSolution One Total Knee Application includes the only commercially available active robot for total knee arthroplasty (TKA) utilizing an open implant library, supporting a variety of implant options. The first generation TSolution One Total Knee Application received FDA 510(k) clearance in October 2019. The second-generation system received FDA clearance in November 2020 and is commercially available in the United States. The core technology of the TSolution One has been used in thousands of successful total joint replacements worldwide.
THINK Surgical actively collaborates with healthcare professionals around the globe to refine our orthopedic products, improving the lives of those suffering from advanced joint disease with precise, accurate, and intelligent technology. Please refer to the instructions for use for the TSolution One Total Knee Application for a complete list of indications, contraindications, warnings, and precautions. For additional product information, please visit www.thinksurgical.com.
THINK Surgical, TSolution One, TPLAN and TCAT are registered trademarks of THINK Surgical, Inc. ©2021 THINK Surgical, Inc. All rights reserved.
About Ortho Development® Corporation
Ortho Development Corporation was founded in 1994 and is a privately held company located in Draper, Utah, U.S.A. The company’s majority owner is Japan Medical Dynamic Marketing, Inc. (Japan MDM), a Japanese medical device distributor. Japan MDM is publicly traded on the First Section of the Tokyo Stock Exchange, ticker 7600.
Ortho Development designs, manufactures, and distributes orthopedic implants and related surgical instruments for the global market, with a primary emphasis on the United States and Japan, and recent expansion into China and Australia.
The company’s principal product focus is total knee and hip joint replacement, but also develops trauma fracture repair and spine treatment products for the Japanese market. Ortho Development products are distributed through a network of independent sales reps and distributors in the United States, and through a direct Japan MDM salesforce in Japan. Surgeons are using Ortho Development products at hundreds of locations, including world-renown orthopedic hospitals and teaching universities, as well as in a widespread number of regional and community hospitals, and ambulatory surgical centers.
Balanced Knee® and BKS TriMax® are registered trademarks of Ortho Development Corporation.
| Sheri Hensley | John Yrungaray |
| SHensley@thinksurgical.com | John.yrungaray@orthodevelopment.com |
| 510-602-0951 | 801-553-9991 |
SOURCE THINK Surgical, Inc.